Dotinurad Related Impurities
Time:2025-07-18
Views:113
Dotinurad, a novel, highly selective URAT1 inhibitor, is primarily indicated for the treatment of gout and associated hyperuricemia.
Gout is a crystal-induced arthropathy caused by the deposition of monosodium urate crystals in the joints. It is directly linked to hyperuricemia resulting from purine metabolism disorders or reduced uric acid excretion. Clinical manifestations include persistent joint swelling, tenderness, deformity, and functional impairment. In recent years, due to changes in dietary patterns and lifestyles, the prevalence of gout in China has shown an increasing and younger-onset trend.
Current pharmacological agents for controlling serum uric acid levels include allopurinol, benzbromarone, febuxostat, and the novel URAT1 inhibitor dotinurad. Dotinurad was jointly developed by Fuji Yakuhin Co., Ltd. and Mochida Pharmaceutical Co., Ltd., and received its initial market approval in Japan in 2020. In December 2024, the NMPA (National Medical Products Administration) officially approved the marketing of dotinurad in China by Eisai China Inc., indicated for gout with hyperuricemia.
As a novel, highly selective URAT1 inhibitor, dotinurad offers significant advantages over traditional agents in both efficacy and safety for managing gout and hyperuricemia.
Dotinurad specifically inhibits the renal URAT1 transporter protein, blocking uric acid reabsorption and promoting its excretion. Crucially, it does not interfere with other transporters (e.g., OAT1/3, ABCG2). This selectivity avoids the side effects common with traditional uricosuric agents caused by non-selective inhibition. Key safety benefits include:
No hepatotoxic metabolites
Low incidence of abnormal liver function with long-term use
Reduced burden on liver and kidneys
Absence of associated cardiovascular risks
Wide applicability with favorable safety profile even for patients with mild-to-moderate renal or hepatic impairment.
Although clinical trial results for dotinurad are promising, its clinical application period remains relatively short. Compared to well-established traditional therapies, further research is needed to comprehensively explore dotinurad‘s long-term safety and efficacy profile.
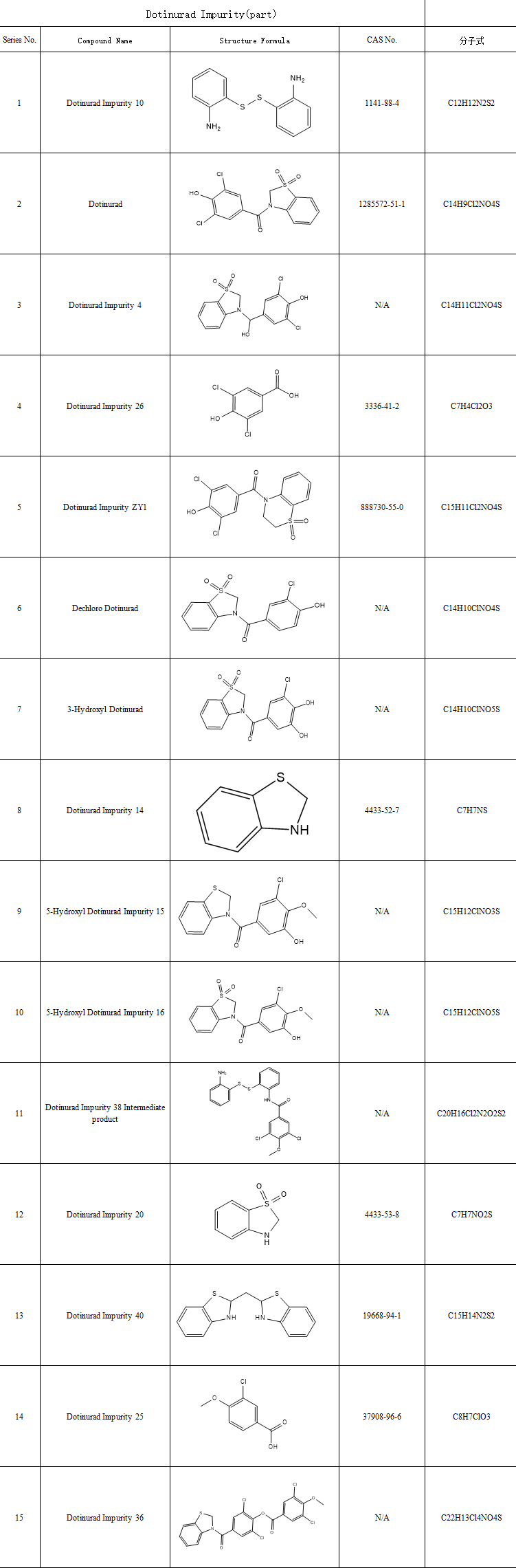
As a novel urate-lowering drug, impurity control is a critical step in ensuring its pharmaceutical safety. Dotinurad-related impurities primarily stem from synthesis process byproducts and degradation during storage. Common Dotinurad impurities include benzoic acid derivatives, benzothiazolone derivatives, hydrolysis byproducts, and isomer impurities.
SZEB supplies the complete range of Dotinurad Related Impurities in stock, such as:Dotinurad Impurity 26 (3,5-Dichloro-4-hydroxybenzoic Acid, CAS 3336-41-2)、Dotinurad Impurity 16 ((1,1-Dioxidobenzo[d]thiazol-3(2H)-yl)(3,5-dichloro-4-methoxyphenyl)methanone, CAS 1285573-44-5) and more.
All products are guaranteed for purity and accompanied by comprehensive supporting documentation, including HPLC, NMR, and MS spectra, along with Certificates of Analysis (COA). Additional analyses such as TGA, QNMR, and 13C-NMR are available upon request.For detailed impurity information, please visit our website: www.ex-biotech.com ,contact us by e-mail : sales@ex-biotech.cn .






.jpg) Wechat
Wechat