Clarithromycin Impurities
Time:2025-03-31
Views:139
Clarithromycin, the star drug in the macrolide antibiotic class, has quickly become a go-to choice in the field of anti-infectives since its successful development by Taisho in Japan back in 1984.
First hitting the market in Ireland and Italy in 1990, clarithromycin stood out thanks to its high stability against stomach acid and a 55% bioavailability. The following year, it got the green light from the US FDA, paving the way for its global rollout. By 1993, it had made its way into the Asian and European markets, reaching over 50 countries.
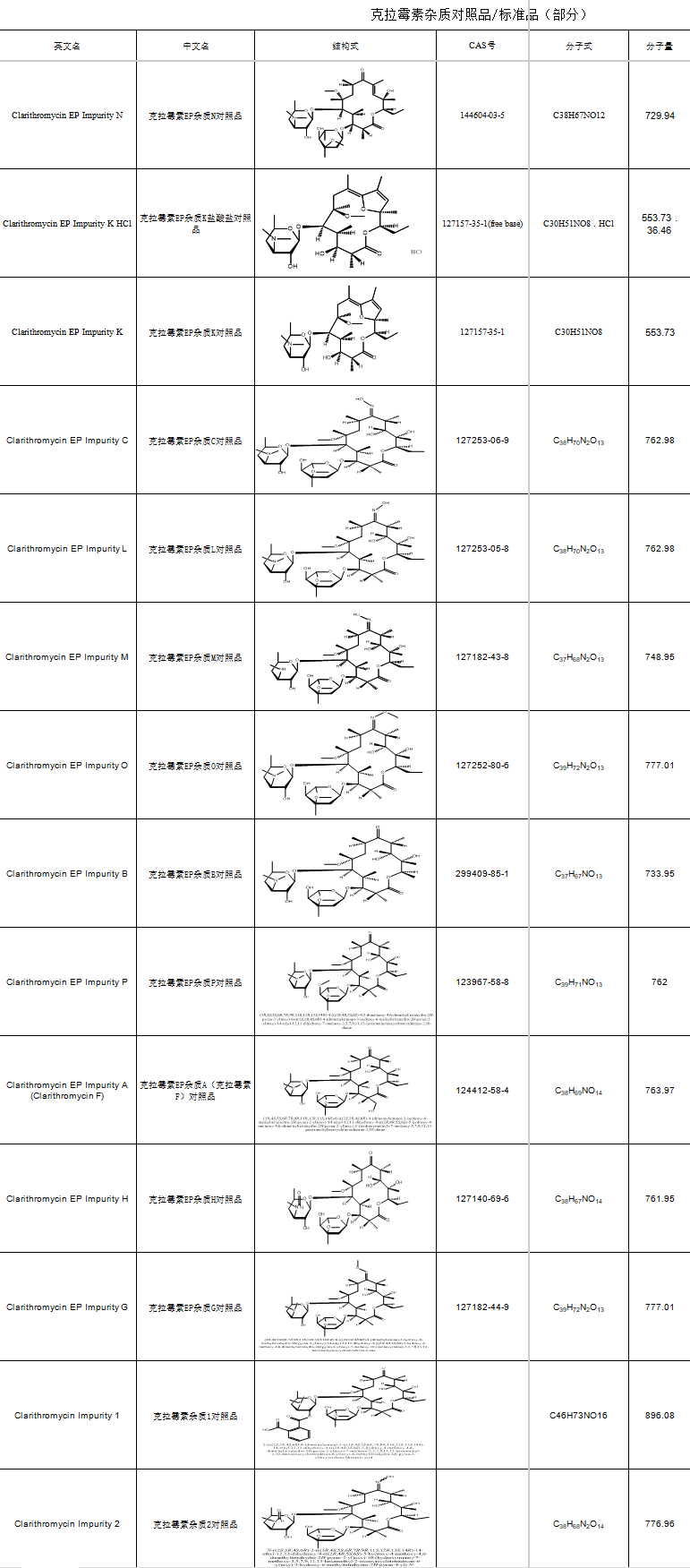
These days, clarithromycin’s broad-spectrum antimicrobial activity and drug resistance issues are becoming more pronounced, yet the demand in emerging markets keeps growing. The clarithromycin molecule, with its multiple hydroxyl groups, is prone to generating a variety of impurities (like hydroxymethylation by-products) during synthesis. This directly impacts the purity and safety of the drug. Common impurities include:the EP impurity series (e.g., EP-P, CAS: 123967-58-8), and impurities 03/04 (CAS: 127253-06-9, 101666-68-6), among others.
High-purity clarithromycin impurity reference substances are crucial for pharmaceutical companies when it comes to R&D, production, and quality control management. Choosing a reliable impurity supplier not only speeds up the R&D process but also ensures compliance and boosts the competitiveness of pharmaceutical firms.
SZEB has a full range of clarithromycin impurities in stock, including the clarithromycin EP series, (14S)/(14R)-hydroxyclarithromycin, clarithromycin N-oxide, and more, to meet different experimental needs. For more information on impurities, check out the official website: www.ex-biotech.com. You can also call the inquiry hotline at 0755-23051186 to get the clarithromycin impurity list and explore more customized solutions.






.jpg) Wechat
Wechat