Iopromide impurities
Time:2025-03-04
Views:158
Iopromide, as a representative of non-ionic iodine contrast agent, has become a “powerful assistant” for precise clinical diagnosis by improving the contrast between diseased and normal tissues. Due to its low osmolality, high tolerance and applicability to newborns of FDA, it has become the preferred choice for CT enhancement, cardiovascular imaging and other scenarios. contrast agent.
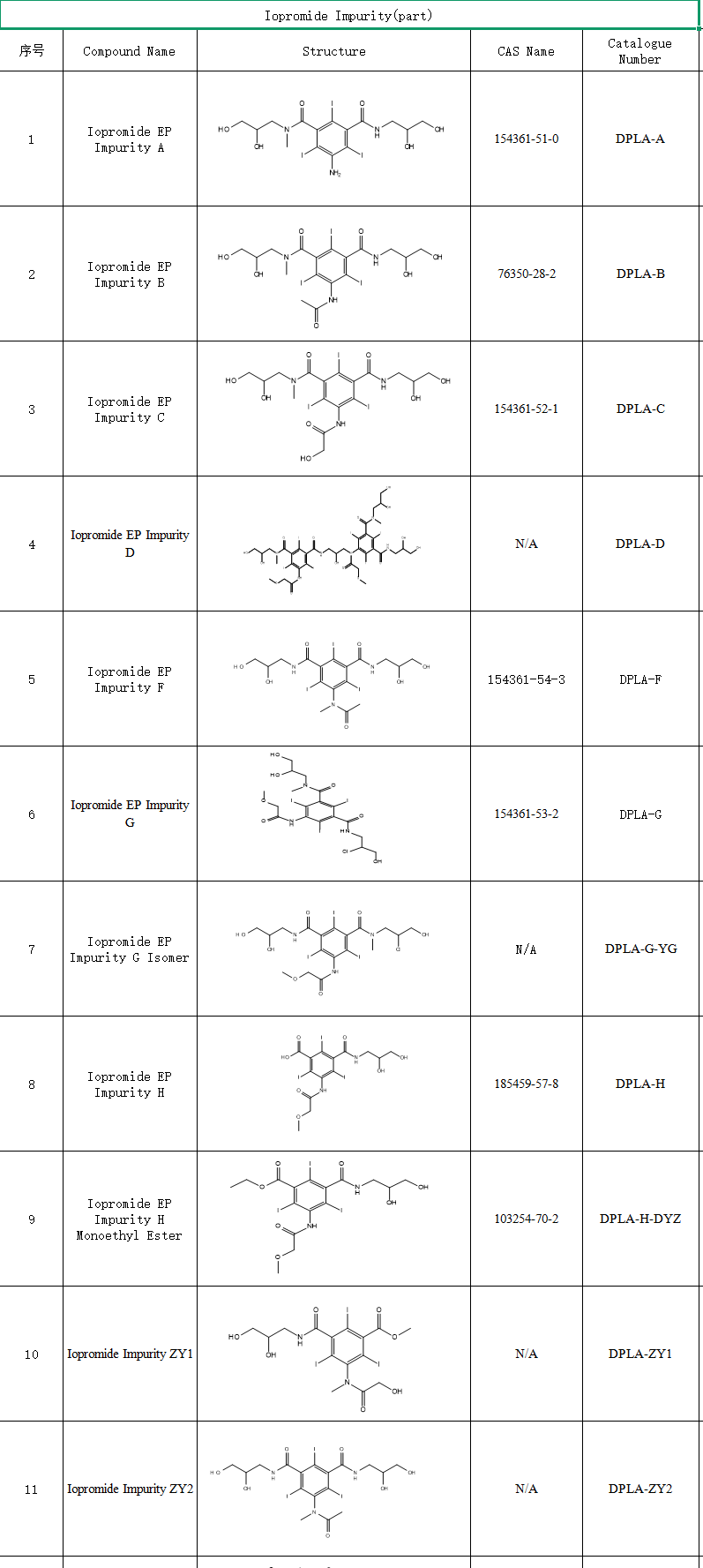
However, the synthesis process of iopromide is complex and prone to produce a variety of isomers and degradation impurities . These impurities may affect drug stability, safety and efficacy, and even trigger allergic reactions or toxicity risks.
Iopromide was approved in China in 1997, and has been occupying the market for a long time since then. 2022, Chengdu Brillaian Pharmaceuticals was approved as the first generic, breaking Bayer AG‘s monopoly; in 2024, Chiatal TianQing became the second domestic approved company. As of 2025, Chongqing ShengHuaXi Pharm, SiChuang KeLun Industry Group and other companies have submitted generic applications, domestic generic alternative competition pattern is obvious.
Iopromide synthesis process is complex, involving multi-step esterification and amidation reaction, preparation, as of March 2025, there are 12 enterprises of iopromide injection through consistency evaluation. Currently, by optimizing the purification process to reduce impurities, it can improve the pass rate of generic drug consistency evaluation.
SZEB provides EP/USP certified impurities , isomers, degradation products, etc., providing complete COA, MS/NMR profiles to meet the requirements of FDA/EMA filing. Visit the official website of Shenzhen Excellence Pharmaceutical: www.ex-biotech.com to get a full set of impurity information, inquiry hotline: 0755-23051186.






.jpg) Wechat
Wechat