liraglutide imourity
Liraglutide is a human glucagon-like peptide-1 analogue (GLP-1 analogue) that lowers blood glucose by selectively binding to and activating the GLP-1 receptor to stimulate insulin secretion and reduce glucagon secretion.
Liraglutide was approved by the FDA for marketing in the United States in 2010. It is indicated for blood glucose control in adult patients with type 2 diabetes and can be used alone or in combination with other oral hypoglycemic agents.In 2014 and 2015, the original liraglutide injection was approved for marketing by the FDA and the EMA, respectively, under the trade name of Saxenda®, for the treatment of obesity or overweight.In March 2023, East Pharm’s liraglutide injection was approved by the In March 2023, Liraglutide Injection of East Pharm was approved by the State Drug Administration for the control of blood glucose in adult patients with type 2 diabetes mellitus, and Liraglutide Injection for the indication of obesity or overweight was approved by the marketing application in July the same year.
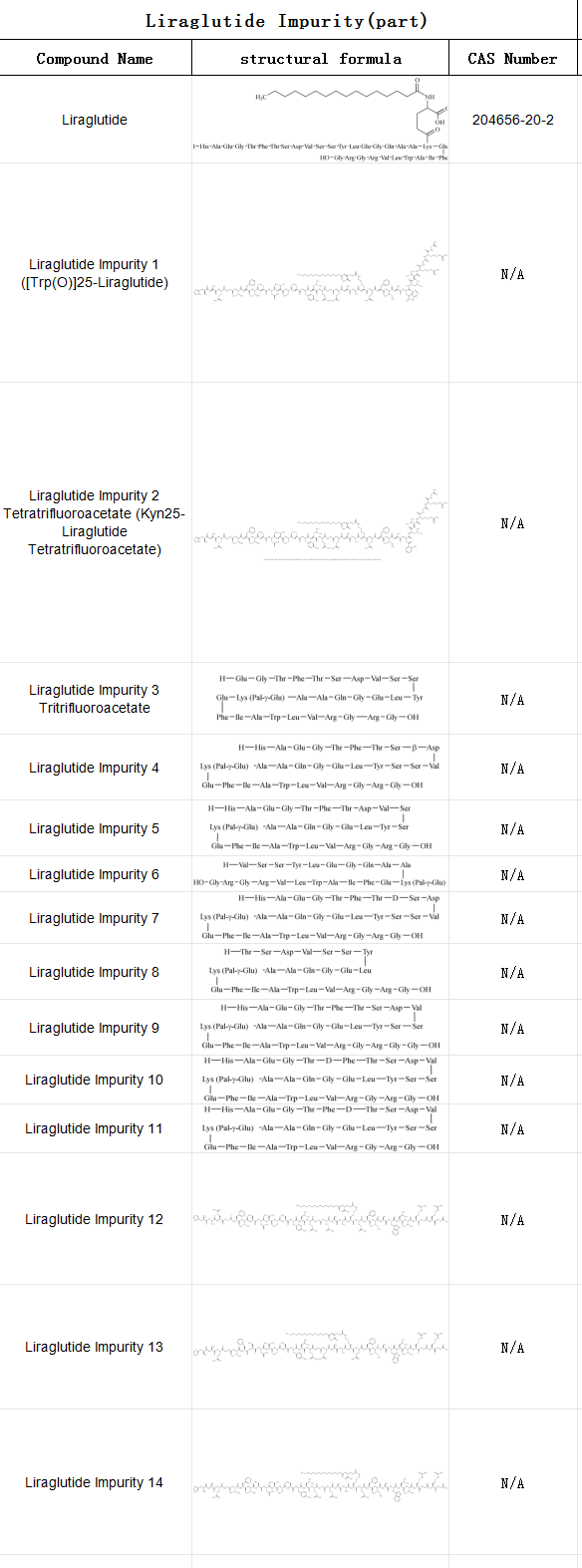
At present, there are many domestic enterprises layout liraglutide biosimilars, SZEB in the supply of liraglutide impurities, for the enterprise for the enterprise‘s active pharmaceutical ingredients and pharmaceutical research and other analytical needs to provide a comprehensive solution. The following is the catalogue of liraglutide impurities (part), more impurity details please visit the official website:






.jpg) Wechat
Wechat