Nitrosamine impurities
Nitrosamine impurities are compounds containing N-nitroso structures that are highly carcinogenic. The problem of contamination with such impurities has spread from valsartan to a wide range of drugs and may arise in a number of processes, including raw materials, formulation, production and packaging.
Nitrosamine impurities can be divided into three categories:
- Small dialkyl nitrosamines, such as NDMA and NDEA, which typically originate from reagents or solvents used in the manufacture of APIs, or from the degradation of products containing secondary amine groups
- Nitrosamine impurities directly related to the drug substance (DSNIs)
- Nitrosamine impurities (RSNIs) associated with excipients or solvents, which may be formed during drug synthesis or excipient manufacturing.
To effectively control and remove nitrosamine impurities from drug manufacturing, the risk of nitrosamine contamination or formation in APIs and finished formulations must be comprehensively assessed.
Global drug regulatory agencies, including China, the United States and the European Union, have issued guidelines on nitrosamine impurity risk assessment, analytical methods and control limits, etc. In September 2024, the US FDA updated its guidance on "Control of Nitrosamine Impurities in Human Drugs”,recommending that APIs and drug manufacturers take steps to detect and prevent unacceptable levels of nitrosamine impurities in their drugs.
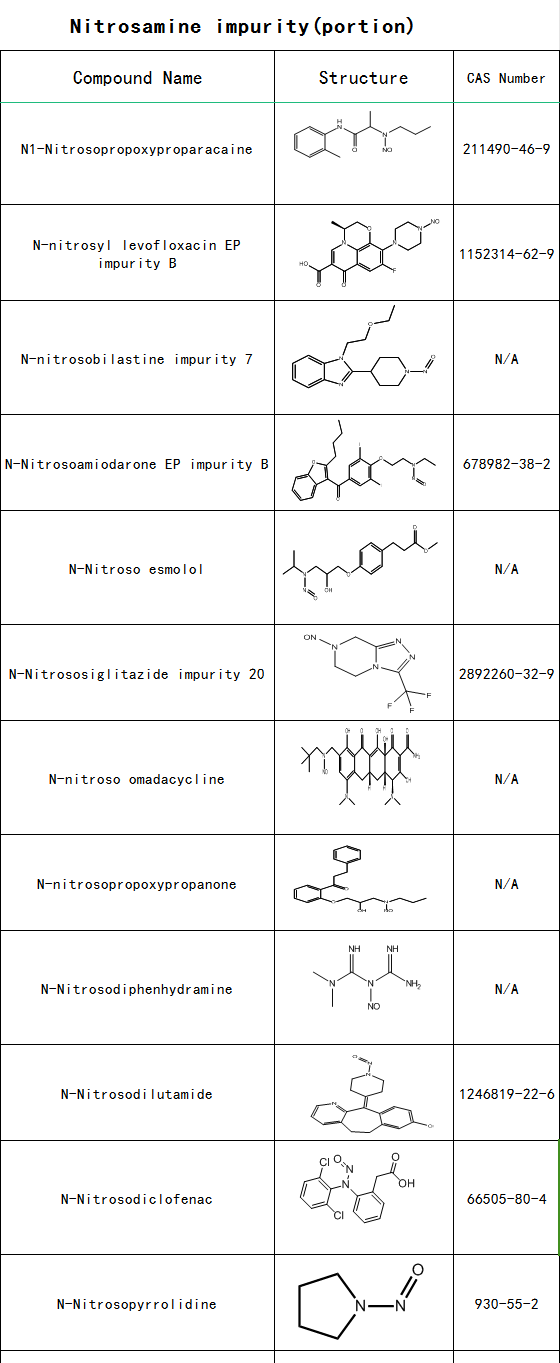
SZEB supplies nitrosamine impurity , and the following is part of its catalogue:






.jpg) Wechat
Wechat