New Product--Segmalutide Impurity
semaglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist, which promotes insulin secretion, inhibits glucagon secretion, delays gastric emptying and increases satiety, and reduces appetite by inhibiting the action of the hypothalamic ingestion centre to achieve the effect of hypoglycemia and weight reduction.
In 2017, injectable semaglutide (weekly formulation) was approved for marketing in the United States for glycaemic control in adult patients with type 2 diabetes mellitus as an adjunctive therapy for adult patients with type 2 diabetes mellitus on the basis of dietary control and exercise, and since then, injectable semaglutide has been marketed in the European Union and Japan.
In 2021, the FDA approved a new indication for injectable semaglutide: for weight management in obese or overweight adults, and in December of the same year, the European Union EMA approved a new indication for WEGOVY for weight management in adults who are obese or overweight.In 2022, the FDA approved the expansion of the indication for WEGOVY to include weight loss in adolescents over 12 years of age.
The patent for the sequence structure and use of semaglutide will expire in March 2026 in China, and dozens of companies, including United Laboratories, East China Pharm,Livzon Pharmaceutical Group Inc, Qilu Pharmaceutical, and CSPC Pharma , have started the filing of semaglutide.
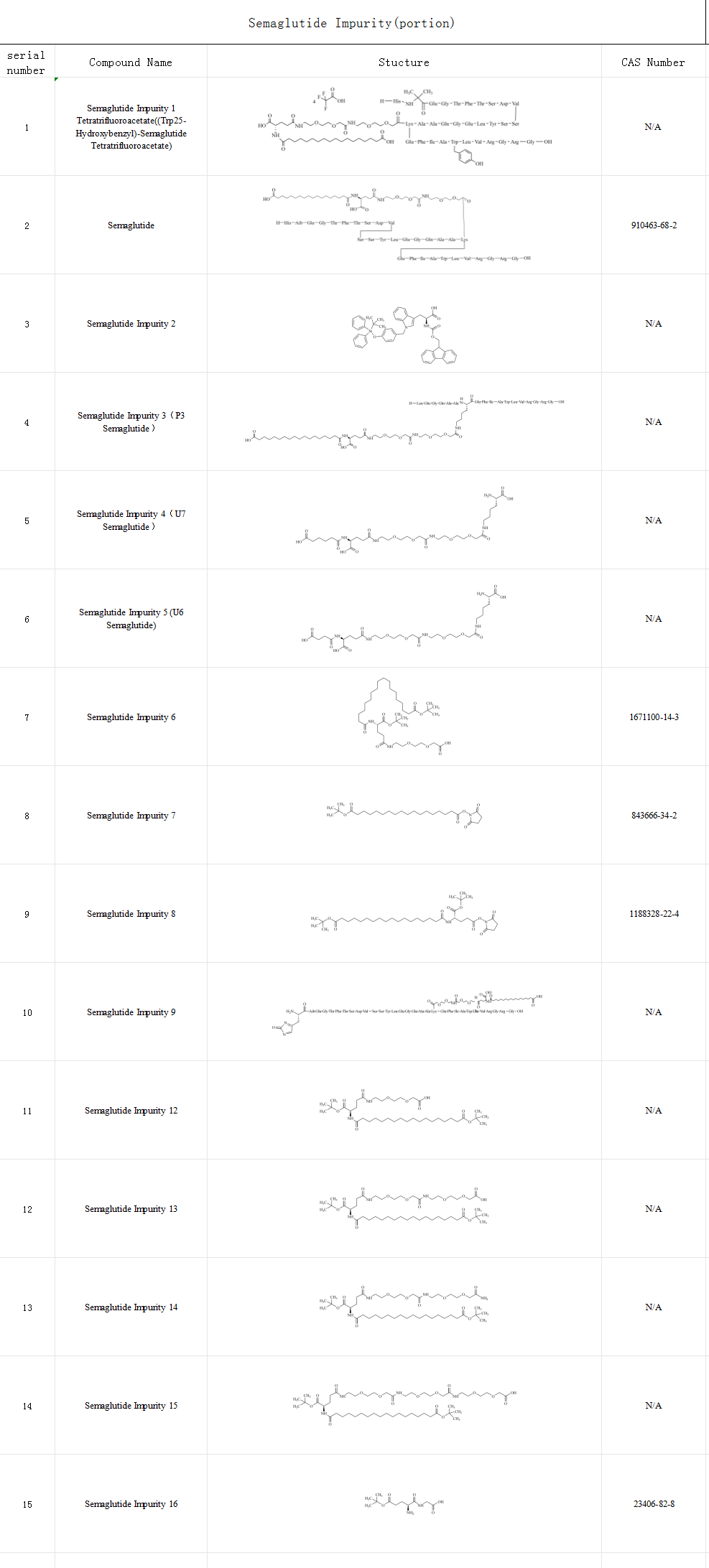
SZEB supplies semaglutide impurities and provides comprehensive solutions for companies‘ analytical needs such as active pharmaceutical ingredients and pharmaceutical research. Below is the catalogue of impurities of semaglutide (part), more impurities details are welcome to visit the official website: www.bio-tech.com .






.jpg) Wechat
Wechat