Nimodipine - Drug Impurity | SZEB
Time:2023-08-30
Views:98
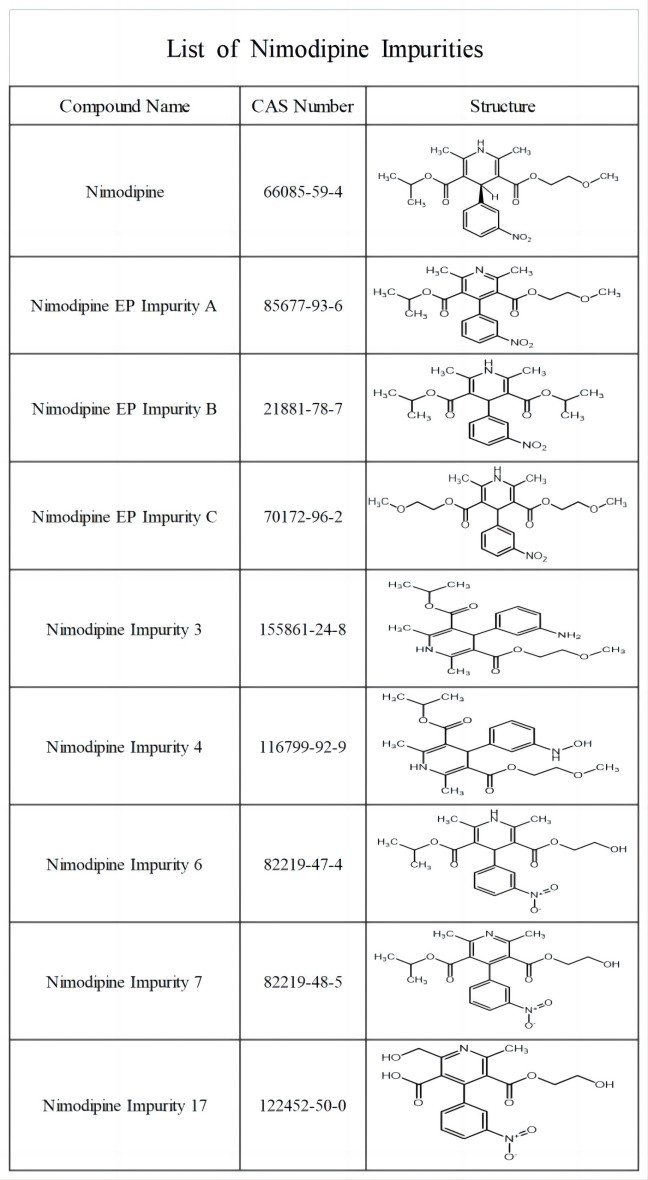
Nimodipine is a second-generation 1,4-dihydropyridine calcium channel blocker. The FDA first approved it for marketing in 1988. Its use is mainly limited to the treatment of vasospasm after subarachnoid hemorrhage.
To support your analysis and help ensure the accuracy of quality processes, we supply a range of Nimodipine-related impurities standards with certified COA and characterization data like Mass, HPLC, NMR & TGA report.
The following are some of the impurities of Nimodipine:






.jpg) Wechat
Wechat