LEQEMBI IQLIK™, the first-ever at-home subcutaneous injection for Alzheimer‘s disease, has been approved.
Alzheimer‘s Disease (AD) isA degenerative disease of the BRAIN characterized by the insidious onset of DEMENTIA. Impairment of MEMORY, judgment, attention span, and problem solving skills are followed by severe APRAXIAS and a global loss of cognitive abilities. The condition primarily occurs after age 60.
The onset of Alzheimer‘s disease is subtle and progresses over several years, generally divided into stages based on severity
1.Mild Stage: The primary manifestation is recent memory loss, such as forgetting things that just happened or were said. Judgment declines, but individuals can generally manage basic self-care.
2.Moderate Stage: Memory deterioration worsens, and long-term memory begins to be affected. Language difficulties, visuospatial decline (e.g., getting lost in familiar places), and significant behavioral and psychiatric abnormalities may appear, requiring assistance with daily living.
3.Severe Stage: Patients become completely dependent on caregivers, experience severe memory loss, may become incontinent, develop limb rigidity, and ultimately may die from complications like infections.
Currently, there is no cure for Alzheimer‘s disease. Treatment aims to slow disease progression, improve symptoms, and enhance quality of life. Besides targeted monoclonal antibodies like Lecanemab and Donanemab, other treatment drugs include cholinesterase inhibitors such as Rivastigmine and Donepezil, which are used to improve cognitive function in patients with mild to moderate AD .
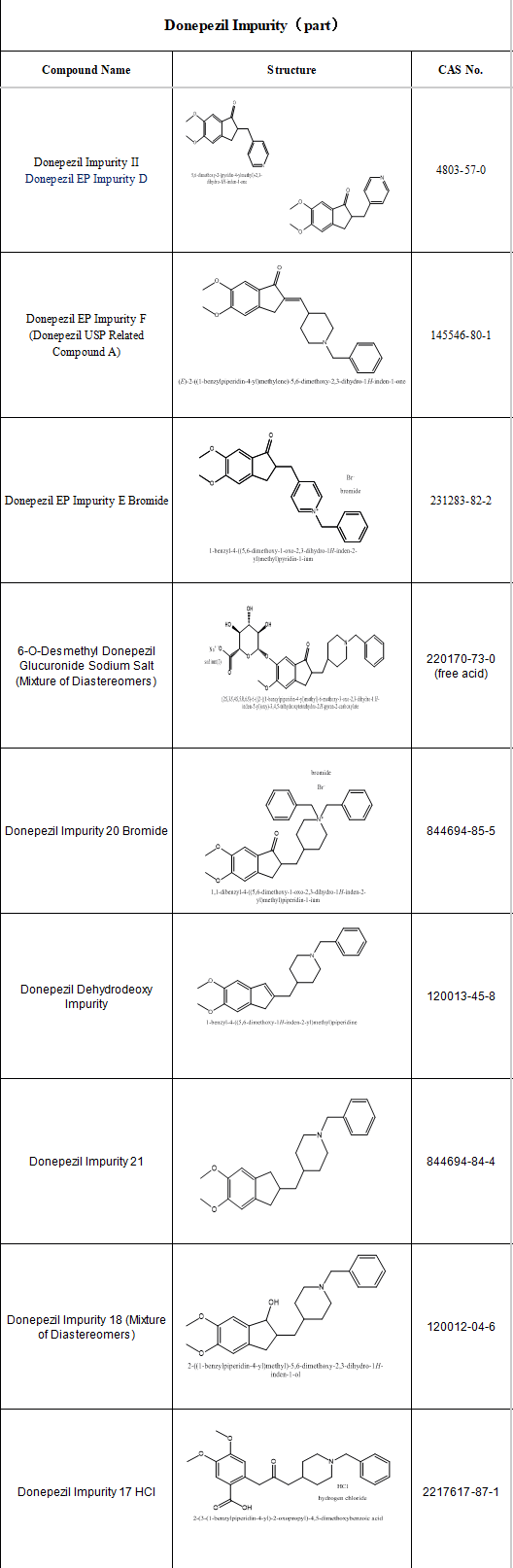
Whether for innovative or generic drugs, impurity research is a critical segment in ensuring drug safety, efficacy, and quality control. Strict testing and analysis must be conducted during drug R&D and quality control processes.
SZEB supplies high-purity Rivastigmine impurity reference standards and Donepezil impurity reference standards. Additionally, we offer professional custom services based on client requirements, providing accurate benchmarks for drug quality research. Below is part of our catalog.






.jpg) Wechat
Wechat