Vilanterol Impurity——in stock
Time:2025-05-15
Views:136
Vilanterol, a long-acting beta2-adrenoceptor agonist (LABA), is primarily used for the long-term maintenance treatment of chronic obstructive pulmonary disease (COPD). By dilating bronchial airways, it alleviates airflow obstruction, enhances ventilation capacity, and improves lung function. Combination therapies (e.g., with umeclidinium or fluticasone) significantly reduce COPD exacerbation frequency.
Vilanterol selectively activates beta2 receptors on airway smooth muscle, stimulating adenylate cyclase to increase intracellular cAMP levels, thereby inducing muscle relaxation. When combined with long-acting muscarinic antagonists (LAMAs) or inhaled corticosteroids (ICS), it suppresses inflammatory mediator release and amplifies bronchodilation efficacy.
Regulatory Milestones
2013: U.S. FDA approval of the originator combination product (umeclidinium/vilanterol, brand name Anoro® Ellipta®).
2018: Chinese NMPA approval for import under the brand name “Oulexin®,” later added to China’s Category B reimbursement list in 2020.
2023: Fluticasone furoate/vilanterol combination (brand name: Relvar® Ellipta®) approved in China for asthma-COPD overlap syndrome (ACOS).
With over 100 million COPD patients in China and a rapidly growing market, stringent impurity control is critical for drug safety and regulatory compliance.
Vilanterol’s manufacturing involves chiral synthesis and bromination steps, where toxic impurities may arise in key intermediates. These impurities demand strict analytical control with high sensitivity to meet pharmacopeial standards.
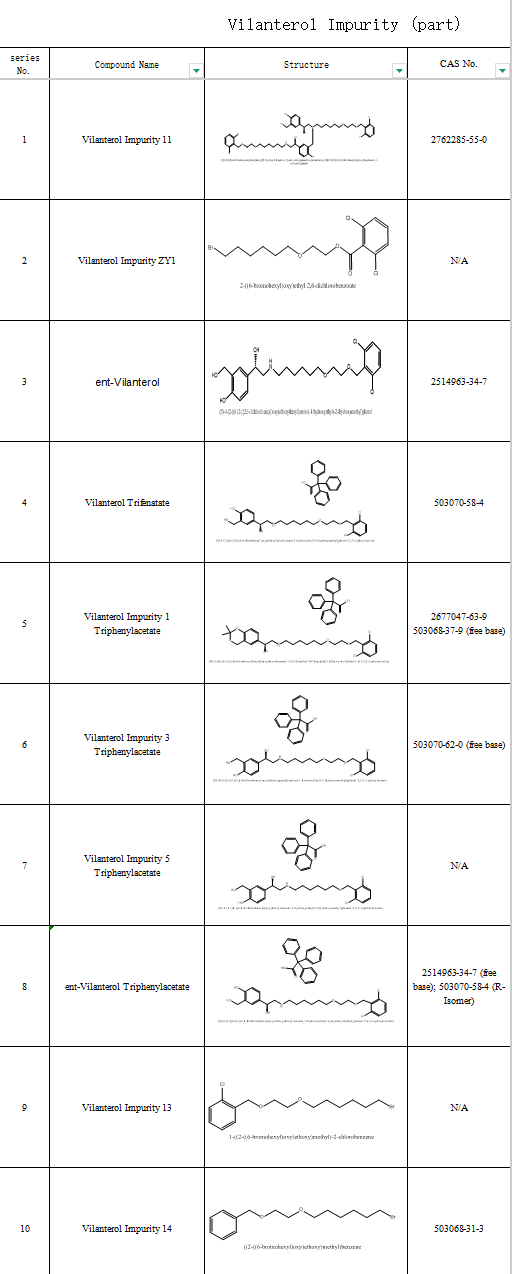
SZEB provides a full suite of vilanterol impurities to accelerate generic drug bioequivalence studies and originator process optimization. Our offerings include:
N-Nitroso vilanterol impurity
Vilanterol enantiomers
Vilanterol triphenylacetate
Comprehensive analytical support includes:COA (Certificate of Analysis)、HPLC purity data, 1H-NMR, and MS.
For impurity specifications and regulatory solutions, visit http://www.ex-biotech.com or contact us at
E-mail:sales@exbiotech.com
E-mail:sales@exbiotech.com






.jpg) Wechat
Wechat