Abemaciclib impurities
Time:2025-04-18
Views:142
Abemaciclib, the third approved CDK4/6 inhibitor worldwide, was developed by Eli Lilly and Co. It has a broad indication covering early adjuvant therapy (32% reduction in risk of recurrence) and first/second line treatment of advanced breast cancer; it can potently inhibit CDK4/6 activity and is 14 times more selective for CDK4 than for CDK6, with much lower hematological toxicity. Since its first approval in the U.S. in 2017, it has rapidly become a core drug in HR+/HER2- breast cancer treatment.
Abemaciclib is approved for marketing in China in December 2020 for patients with HR+/HER2-advanced breast cancer, and will be expanded to adjuvant treatment for early-stage breast cancer in December 2021, with the “Ki-67 ≥ 20%” restriction lifted in 2023 to benefit more high-risk patients.
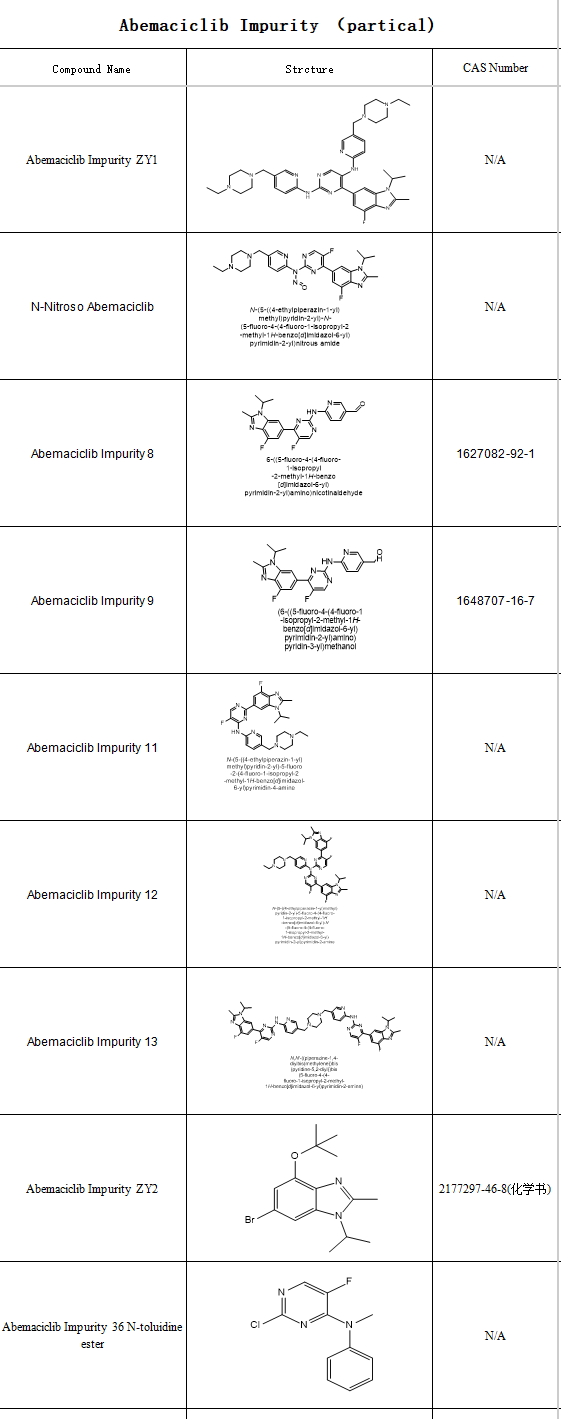
The patent protection period of Abemaciclib in China is until December 2029, generic enterprises have already laid out, impurity research has become a key link in the R&D and production of pharmaceutical enterprises.
SZEB supplies a full set of Abemaciclib impurities in stock, covering Abemaciclib impurity 3, Abemaciclib impurity 6 and more than 10 key impurities, covering most of the synthesis process pathway. We provide COA, HPLC, H-NMR, MS and other full set of analytical reports with the goods, and support C-NMR, TGA and other customized tests. Visit the official website of SZEB at www.ex-biotech.com or contact us at 0755-23051186 to get the full information of Abemaciclib impurities and understand the customized solutions.






.jpg) Wechat
Wechat