Cefcapene Impurity
Time:2025-02-11
Views:137
Cefcapene, as a third-generation cephalosporin antibiotic, has become an important research target in the field of anti-infectives due to its unique chemical structure and broad-spectrum antibacterial activity. Impurities generated during drug production may affect the safety, efficacy and stability of drugs. Therefore, accurate impurity analysis and control is the core link of drug development and production.
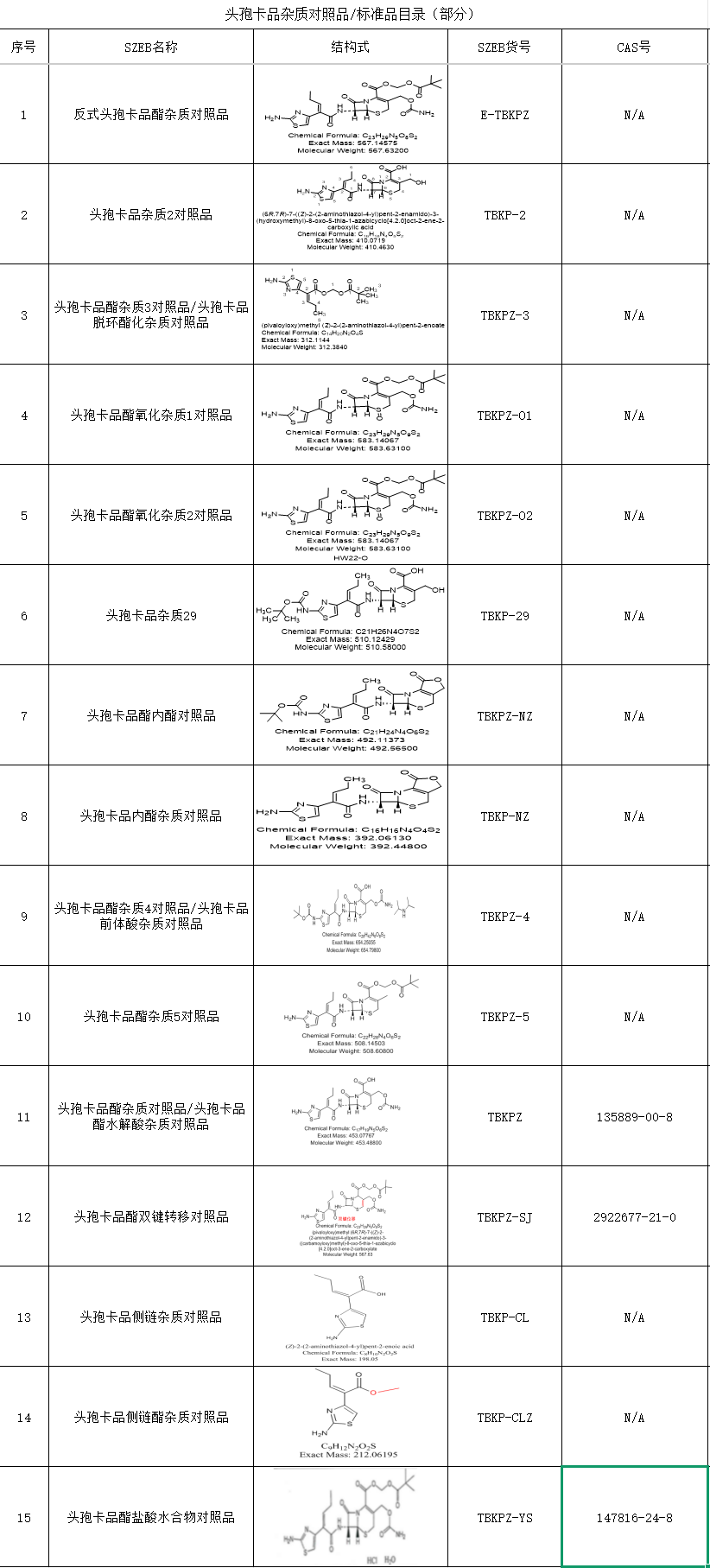
SZEB provides a full set of Cefcapene main components (CAS 135889-00-8), dimers, isomers and more than 20 known impurity controls, covering pharmacopoeia requirements and corporate internal control standards, with complete COA (Certificate of Analysis), Mass Spectrometry (MS), Nuclear Magnetic Resonance (NMR) and HPLC profiles.
Below is the Cefcapene impurity spot catalog, for more information, please visit the official website or contact us - Tel: 0755-23051186/Email: sales@exbiotech.com for the complete product list.






.jpg) Wechat
Wechat