Ezetimibe impurity
Ezetimibe reduces blood cholesterol levels by inhibiting cholesterol absorption in the small intestine. It has been shown that the molecular target of ezetimibe is the sterol carrier, which is implicated in the intestinal absorption of cholesterol and phytosterols. Ezetimibe attaches to the brush border of the villous epithelium of the small intestine. It inhibits the absorption of cholesterol, thereby decreasing the transport of cholesterol from the small intestine to the liver, resulting in a decrease in hepatic cholesterol stores and thus increasing the removal of cholesterol from the blood. The main preparations of ezetimibe are ezetimibe tablets with the following indications:
1. Primary hypercholesterolemia, as an adjunctive therapy to dietary control, alone or in combination with HMG-CoA reductase inhibitors (statins) for the treatment of primary (heterozygous familial or non-familial) hypercholesterolemia, which reduces total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and apolipoprotein B (ApoB);
2. pure familial hypercholesterolemia (HoFH), in combination with statins, as an adjunct to other lipid-lowering therapies (e.g., LDL-C plasma-split replacement), or to lower TC and LDL-C levels in patients with HoFH when other lipid-lowering therapies are ineffective.
3. Steroidemia (or phytosterolemia), as an adjunctive therapy to dietary control to reduce glutenol and phytosterol levels in patients with familial steroidemia; and
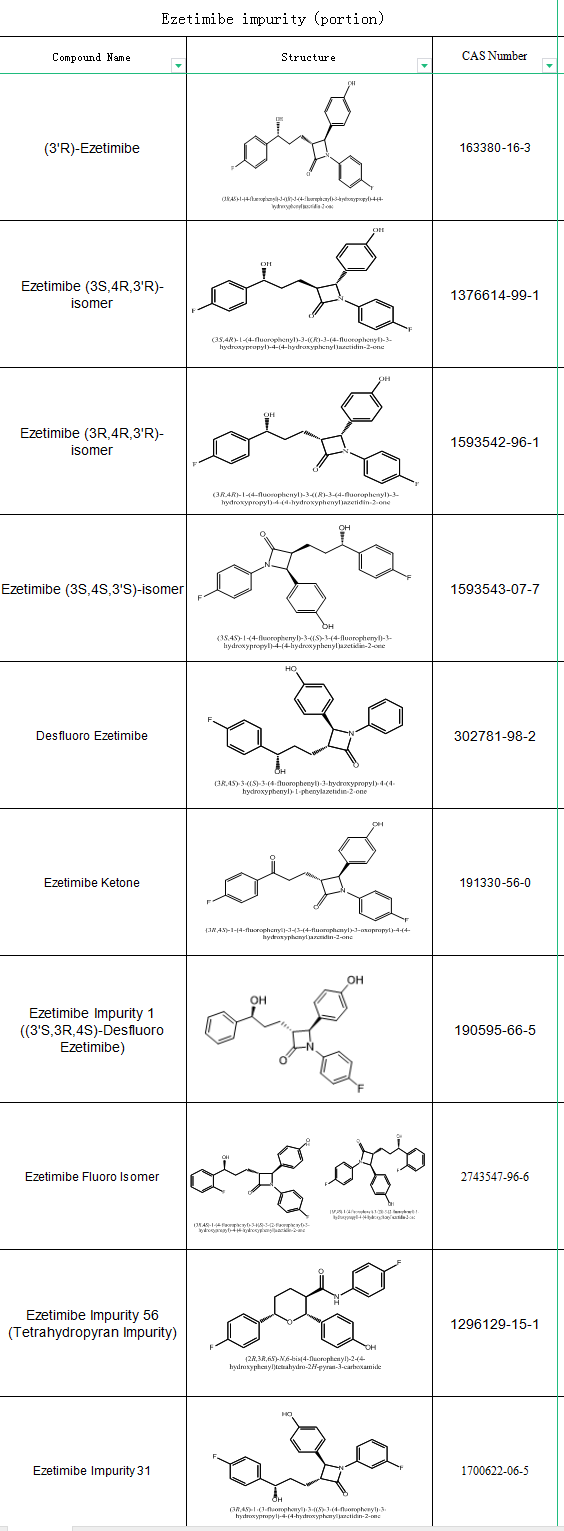
As an important non-statin lipid-lowering drug, Ezetimibe impurity is crucial for research and quality control, SZEB supplies Ezetimibe impurity, which can provide comprehensive solutions for the analytical needs of enterprises in active pharmaceutical ingredients and pharmaceutical research. The catalog (partial) is as follows:






.jpg) Wechat
Wechat