Iohexol--drugs for medical imaging diagnosis
iohexolis a commonly used water-soluble, non-ionic X-CT contrast material, which is widely used in medical imaging examination. It has the advantages of low contrast density, low toxicity, good tolerance, etc. It is one of the best contrast agents at present, and it has completely replaced ionic contrast agents in some countries.
The safety of iohexol itself is high. Acute toxicity evaluations in animal models have shown that iohexol has low acute toxicity, but it may still cause serious adverse reactions under certain circumstances. Prolonged exposure to iohexol may result in adverse reproductive effects, including decreased spermatogenesis and abnormal embryonic development.
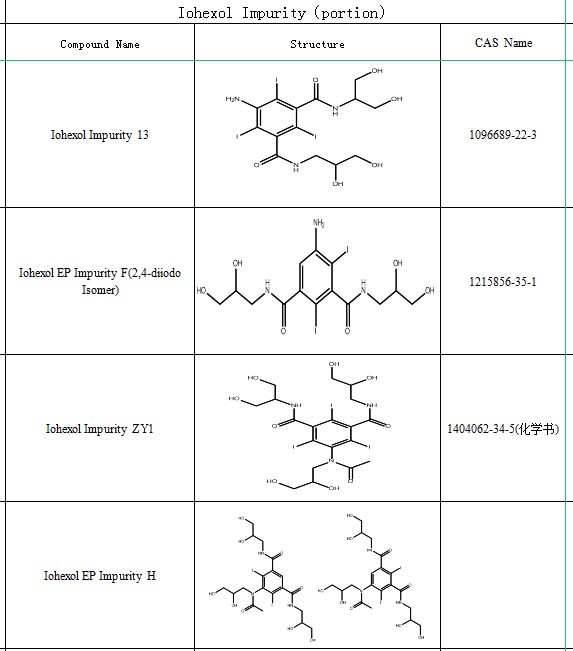
Therefore, research on iohexol impurities pharmaceutical quality control is very critical, most of the current production and quality control of iohexol is based on the European Pharmacopoeia method, iohexol is prone to over-alkylated impurities during the preparation process, the European Pharmacopoeia (EP9.0) also contains four known over-alkylated impurities, while the pharmacopoeia indicates that over-alkylated impurities are controlled by the liquid phase, and the liquid phase is associated with a main peak of the The relative retention time is 1.1 - 1.4, and the limit is that the sum of the four impurities does not exceed 0.6%.
The Chinese Pharmacopoeia 2020 edition also has relevant provisions, between 1.1 to 1.4 times the relative retention time of the peaks of the isomers outside of iodohexol if there is a cluster of impurity peaks of O-alkyl compounds, the sum of the peak areas of the impurities shall not be greater than 0.6% of the total peak area, and the peak area of the other individual impurities shall not be greater than 0.1% of the total peak area, and the sum of the peak areas of the other impurities shall not be greater than 0.3% of the total peak area.
More research on iohexol impurities is not only limited to the detection and quantification of impurities, but also includes preparation methods and potential applications of impurities. SZEB supplies iohexol impurity and is committed to helping users solve drug impurity problems encountered in drug research sessions.
The following is a part of the catalog of iohexol impurity and, more detailed information of impurities can be viewed on the official website——www.bio-tech.com., if you have any needs for medication impurity , please feel free to contact us by e-mail:sales@ex-biotech.com.We are willing to serve you!






.jpg) Wechat
Wechat