Finerenon,a new diabetic nephropathy drug
Finerenone is the world‘s first novel non-steroidal MRA analogue approved for the treatment of type 2 diabetes mellitus-associated chronic kidney disease, bringing new options for the treatment of diabetic nephropathy.
During the preparation of Finerenone, special attention needs to be paid to the control of stereochemical purity, because Finerenone contains chiral centres, and its stereoisomers may have different biological activities and pharmacological effects. In addition, the different crystalline forms of Finerenone should also be considered as one of the impurities to be controlled.
To effectively control the stereochemical purity during the synthesis of Finerenone, several strategies can be adopted:
1. optimise the reaction conditions:
The stereochemistry of the product can be effectively controlled by adjusting parameters such as pH, temperature and time of the reaction medium. For example, higher stereoselectivity and yield can be obtained by performing the elimination reaction under acidic conditions. This indicates that the stereochemical purity of the products can be controlled to some extent by precisely controlling the reaction conditions.
2. Post-processing separation technology
During the synthesis process, the target compounds can be purified by efficient separation techniques such as high performance liquid chromatography (HPLC) or gas chromatography (GC) to improve their stereochemical purity. These techniques can help to isolate high purity chiral compounds from mixtures.
3. Multi-step synthesis strategy
The use of a multi-step synthetic strategy, where stereoselectivity is optimised at each step, allows for the gradual construction of complex structures of target molecules while maintaining high stereochemical purity.
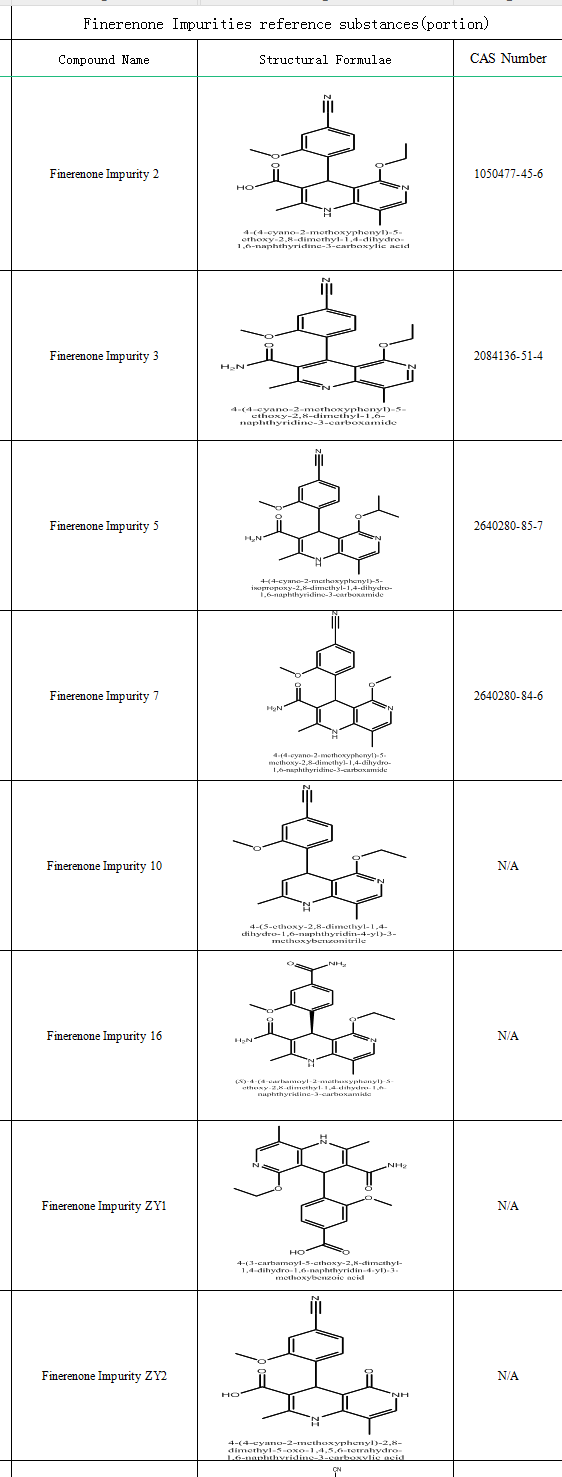
Finerenone has been on the market for a short period of time, and with the accumulation of clinical experience and research evidence, it is expected to bring more benefits to diabetic nephropathy patients in the future. There is not much research on Finerenon impurities in the current literature,d and there are no standards for the detection of Finerenone and its related substances in the current domestic and international pharmacopoeias.
SZEB supplies Finerenone impurity control/standard, the following is part of its catalogue, more information about the drug impurity, please log on the official website: www.ex-biotech.com to view, if you need welcome to contact us,we are willing to serve you!






.jpg) Wechat
Wechat