Enhanced Supervision in Pharmaceutical Regulation
Time:2025-06-25
Views:95
On June 7, the National Medical Products Administration (NMPA) announced a nationwide recall of three batches of commonly used drugs posing significant quality risks.The affected products include:
Sukang Ganmaoling Granules and Keli Ting Oral Solution : Illegally added excessive doses of diclofenac sodium (exceeding the limit by 3.8-fold), which may cause acute liver injury.
Gutongxiao Magnetic Therapy Patches : Process defects resulted in a 210% over-release of diclofenac diethylamine, potentially leading to abrupt blood concentration spikes in patients with pre-existing liver/kidney conditions.
Wen Yaping Tablets (adjunctive antihypertensive drug): Detected with nitrosamine compounds (classified as Group 2A carcinogens by international standards). Animal studies indicate 90-day continuous use may cause renal fibrosis.
This recall—the second major safety intervention in 2025—follows the NMPA’s January alert regarding 13 batches of non-compliant drugs containing visible particles and illegal additives. It reaffirms that impurity control and manufacturing process risks remain critical industry challenges. Pharmaceutical companies must urgently implement such as stringenting raw material controls ,Enhancing process validation protocols and advancing trace-level detection capabilities for emerging carcinogens (e.g., nitrosamines)
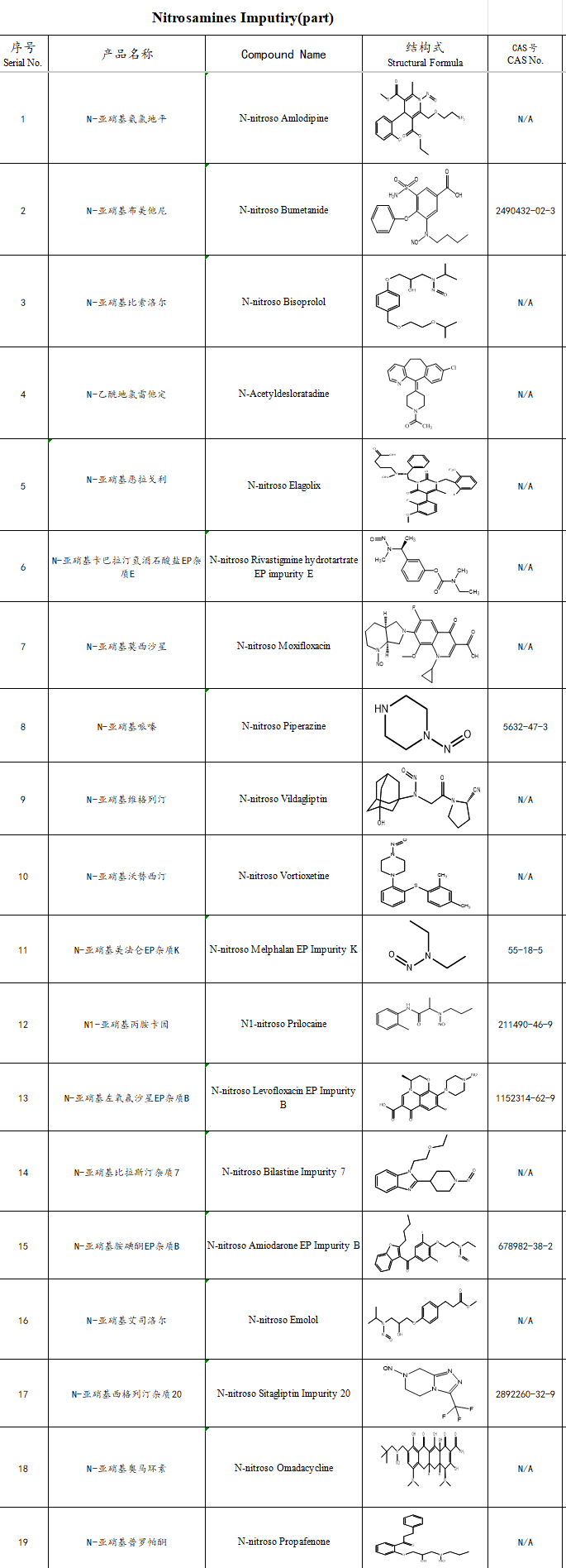
Addressing these technical demands, SZEB supply 5,000+ pharmaceutical impurity reference standards. For the industry‘s heightened focus on nitrosamines, we offer over 20 high-demand compounds, including NDMA and NDEA, empowering rapid compliance with evolving regulations.
Partial Nitrosamine Catalog :
For full specifications, visit our Product Center at http://www.ex-biotech.com. Custom Solutions & Product Quotations:E-mail:sales@ex-biotech.com .






.jpg) Wechat
Wechat