-
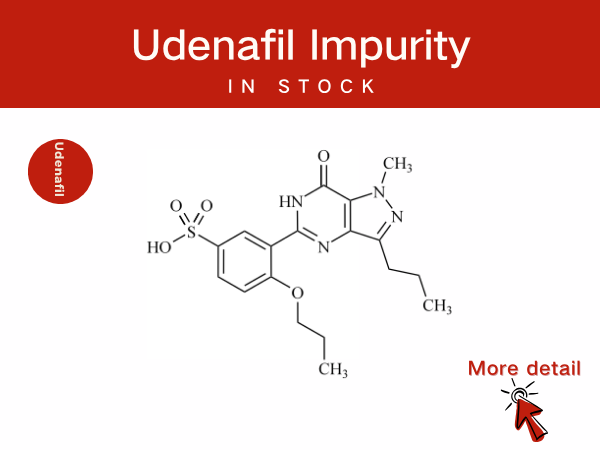
Udenafil Impurity Control and Scientific Innovation: Professional Pharmac...
Udenafil is a selective phosphodiesterase type 5 (PDE5) inhibitor. It is primarily used for the treatment of male erectile dysfunction (ED) and is also applied in the management of premature ej...
See More
-
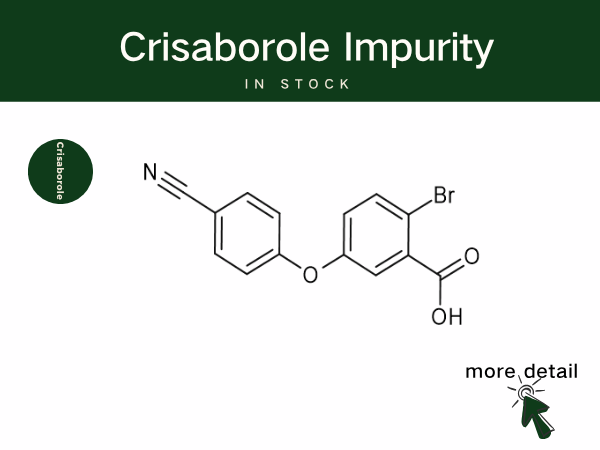
Crisaborole is Reshaping Atopic Dermatitis Treatment, Backed by SZEB Q...
Crisaborole is a boron-containing small-molecule anti-inflammatory drug, classified as a non-steroidal topical phosphodiesterase-4 (PDE-4) inhibitor. By inhibiting PDE-4, the drug increases th...
See More
-
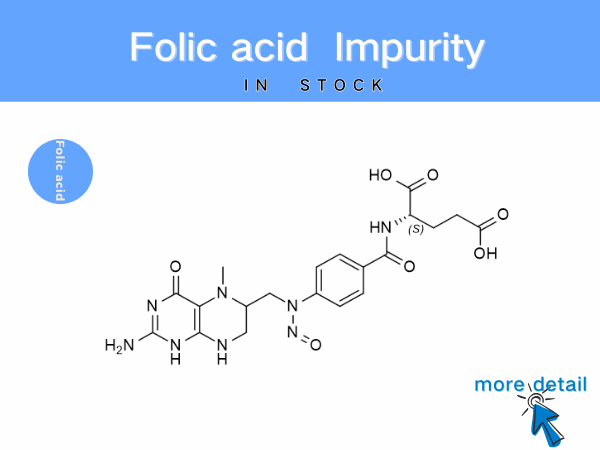
Folic Acid Impurities: Reference Standards for Pharmaceutical Quality Co...
Beyond process-related impurities, factors such as light exposure, high temperatures, or humid conditions can cause the oxidation or degradation of folic acid, leading to the formation of new impurit...
See More
-
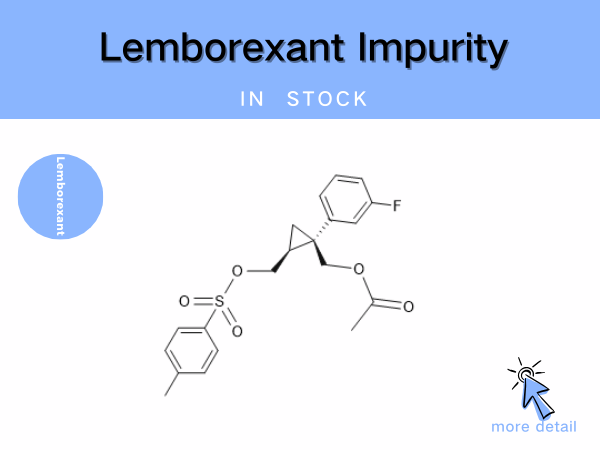
Lemborexant Impurity
Lemborexant is the first dual orexin receptor antagonist (DORA) approved for marketing in China. Developed by Eisai, it was first launched in the United States in December 2019 and received...
See More
-
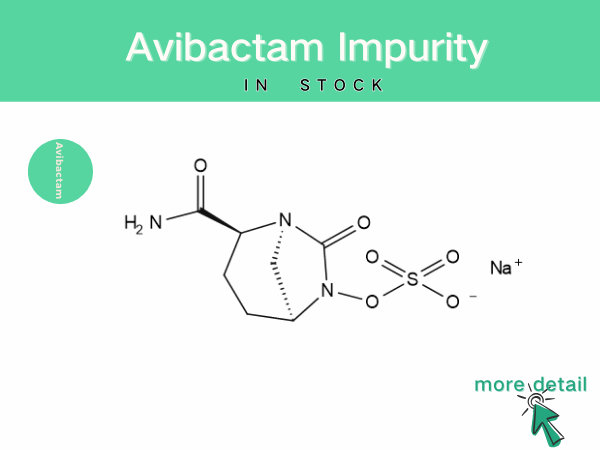
Avibactam impurity
With the widespread application and in-depth research of avibactam, the analysis and control of its impurities and content have become increasingly important. During the synthesis and production of ...
See More
-
SZEB | Continuous Updates on Nitrosamine Impurities...
Facing dynamic regulatory updates and the structural diversity of NDSRIs, Shenzhen Superior Excellence Biotechnology (SZEB) responds rapidly by supplying relevant drug impurity refer...
See More
-
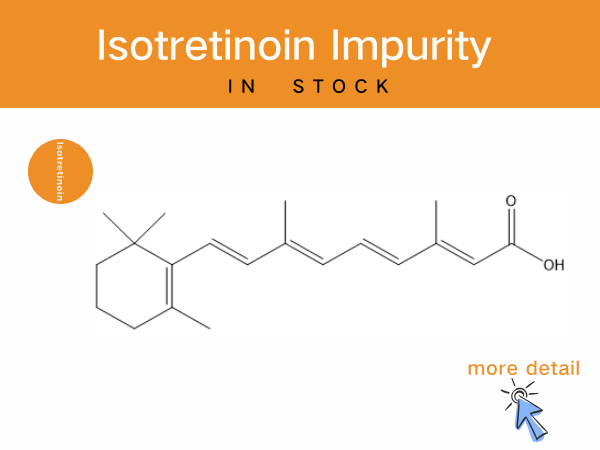
Isotretinoin Impurities: Professional Solutions of Reference Standards for...
As a critical drug for moderate-to-severe acne, Isotretinoin’s production and quality control are subject to rigorous regulatory standards. The Chinese Pharmacopoeia (2025 Edition)mandates that...
See More
-
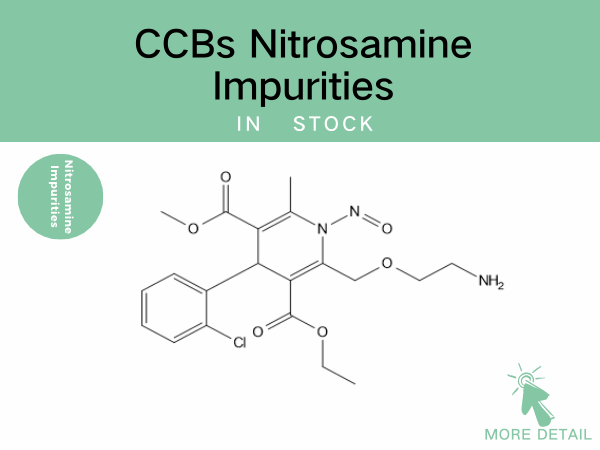
CCBs Nitrosamine Impurities——Precise Drug References Empowering Drug...
Nitrosamine impurity control remains a global regulatory priority. For CCBs—the most widely prescribed antihypertensive class—robust identification and quantification of these impurities are cr...
See More
-
How Does Olopatadine Safeguard Allergy Patients?...
Impurity control is critical for drug efficacy and safety. Olopatadine may generate impurities during synthesis, storage, or metabolism. The Chinese Pharmacopoeia (2025 Edition) mandates limits ...
See More
-
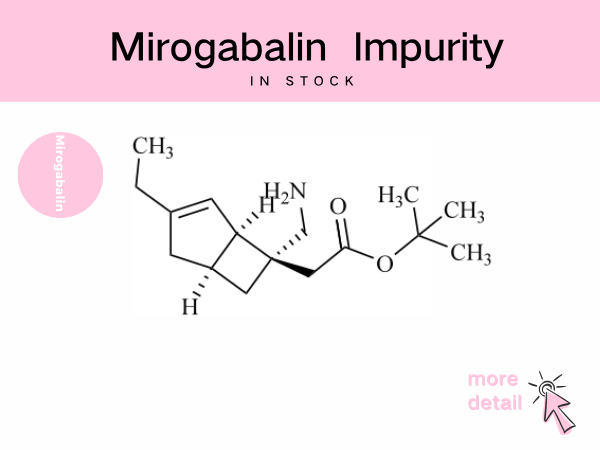
Mirogabalin Impurity ——in stock
The mirogabalin molecule contains multiple chiral centers and unsaturated bonds, rendering it susceptible to generating stereoisomers or positional isomer impurities during synthesis and storage, as ...
See More
-
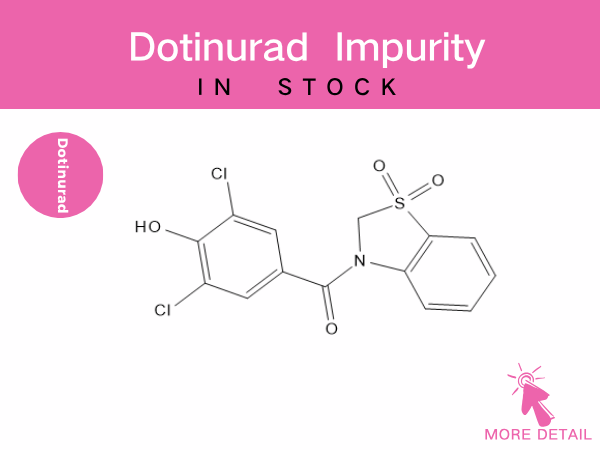
Dotinurad Related Impurities
As a novel urate-lowering drug, impurity control is a critical step in ensuring its pharmaceutical safety. Dotinurad-related impurities primarily stem from synthesis process byproducts and d...
See More
-
Vitamin B6 (Pyridoxine) Impurity
Vitamin B6 is not resistant to high temperatures and is easily destroyed under light and alkaline conditions, with more degradation impurities. Different impurities may also be generated during the...
See More
-
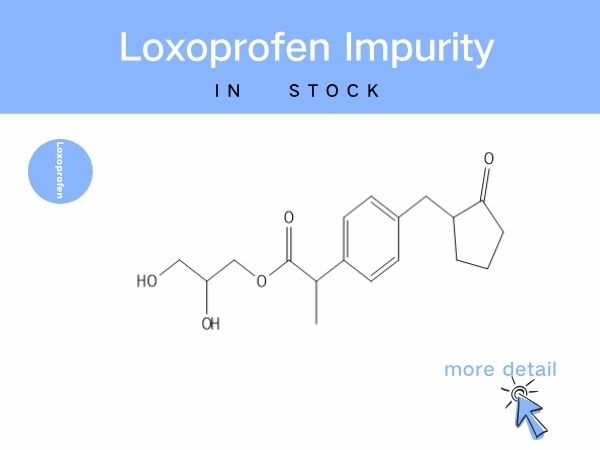
Loxoprofen Impurity
Notably, brominated intermediates from synthesis may carry potential toxicity, while impurities such as ring-opened degradants and glycerol-esterified byproducts can compromise drug e...
See More
-
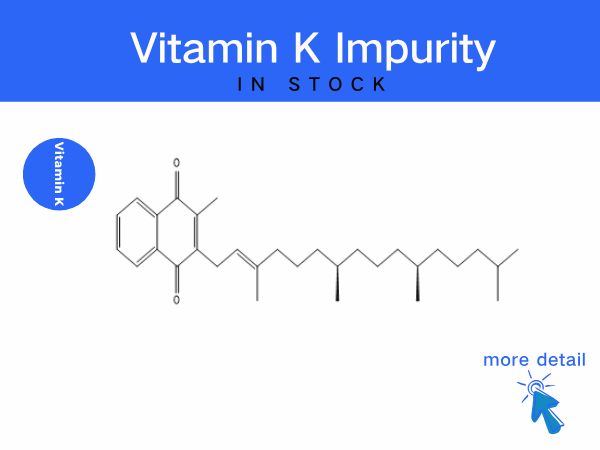
Vitamin K impurity
To address pain points in procurement, SZEB offer a full life-cycle management program: We supplies a complete range of Vitamin K impurities, including:Vitamin K1 Impurity Series...
See More
-
Finerenone impurity
SZEB supplies a comprehensive portfolio of Finerenone impurity reference standards, including stereoisomers such as: (S)-Finerenone、(R)-Finerenone、Finerenone racemate,Below i...
See More
-
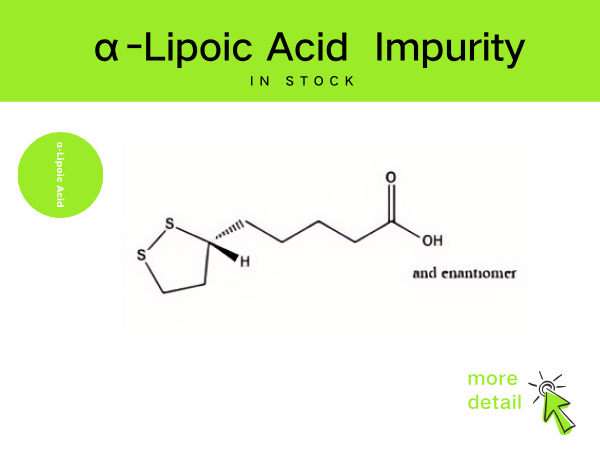
Lipoic Acid Impurity
SZEB supplies a full range of lipoic acid impurities in stock, including the main process impurities in the preparation of lipoic acid, such as: cyclization by-product impurity A , CAS: 12042...
See More
-
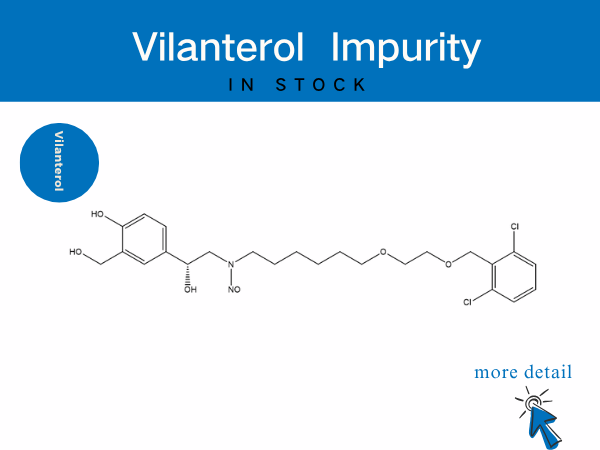
Vilanterol Impurity——in stock
SZEB provides a full suite of vilanterol impurities to accelerate generic drug bioequivalence studies and originator process optimization. Our offerings include: N-Nitroso vilanterol ...
See More
-
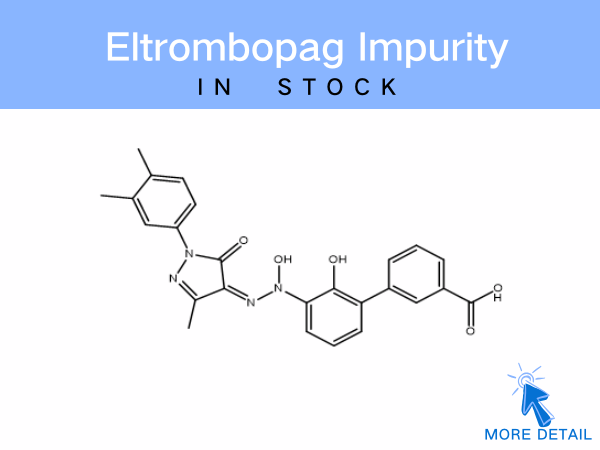
Eltrombopag Impurity
SZEB specializes in supplying a comprehensive range of eltrombopag impurities, including:EP-specified impurities、Eltrombopag olamine、Dimer-related impurities These referenc...
See More
-
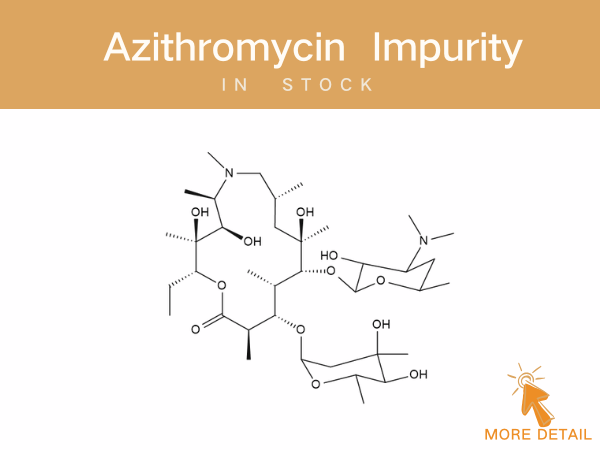
Azithromycin Impurities——in stock
Azithromycin A 11,12-cyclic borate 3’-N-Demethyl-3’-N-(phenylsulfonyl) azithromycin 3-Hydroxy azithromycin acid For detailed impurity profiles and inquiries, visit http://www.ex-biotech.c...
See More
-
Brexpiprazole: A Third-Generation Antipsychotic...
在其实际生产工艺中,会涉及多种布瑞哌唑杂质以及相关研究分析,目前已知部分杂质包括:布瑞哌唑杂质1--50,布瑞哌唑亚砜,结构警示杂质布瑞哌唑氮氧化物等。
See More








.jpg) Wechat
Wechat