Lemborexant Impurity
Eisai China announced that its globally significant insomnia treatment drug, Lemborexant Tablets (trade name: Dayvigo®), has officially received approval from the National Medical Products Administration (NMPA) for marketing. It is indicated for the treatment of adult patients with insomnia characterized by difficulty falling asleep or difficulty staying asleep.
Lemborexant is the first dual orexin receptor antagonist (DORA) approved for marketing in China. Developed by Eisai, it was first launched in the United States in December 2019 and received approval in China on May 20, 2025.Its compound patent is protected until September 2031
.
Insomnia is one of the most common sleep problems. According to the "2025 China Sleep Health Survey Report," over 500 million people in China experience sleep disturbances in 2025
. Data from the China Sleep Research Society indicates that the persistence rate of adult insomnia ranges from 30% to 60%, with nearly half of severe insomnia cases lasting for over ten years
.
Previously, traditional insomnia treatments, such as benzodiazepines, primarily worked by enhancing the inhibitory neurotransmitter GABA in the brain, broadly suppressing the activity of the entire central nervous system to induce sleep
. However, this mechanism of action is often associated with significant side effects, including a high potential for dependence and addiction
. Long-term use may lead to tolerance, reducing efficacy, and discontinuation can result in withdrawal symptoms and rebound insomnia
.
In contrast, Lemborexant‘s mechanism of action involves blocking the "orexin" signal in the brain that promotes wakefulness. It competitively binds to both OX1R and OX2R receptors, inhibiting the state of over-arousal and thus inducing sleep in a more physiological manner.In clinical trials, somnolence was the most commonly reported adverse reaction, with the majority of adverse events being mild or moderate in severity. Lemborexant showed no significant impact on cognitive function or respiratory function, and carries no risk of addiction.
The "Chinese Guidelines for the Diagnosis and Treatment of Insomnia Disorder" (2025) identifies Lemborexant as the optimal drug choice for improving sleep efficiency. It is also recommended as one of the first-line drug categories in the "Chinese Adult Insomnia Diagnosis and Treatment Guidelines" (2023)
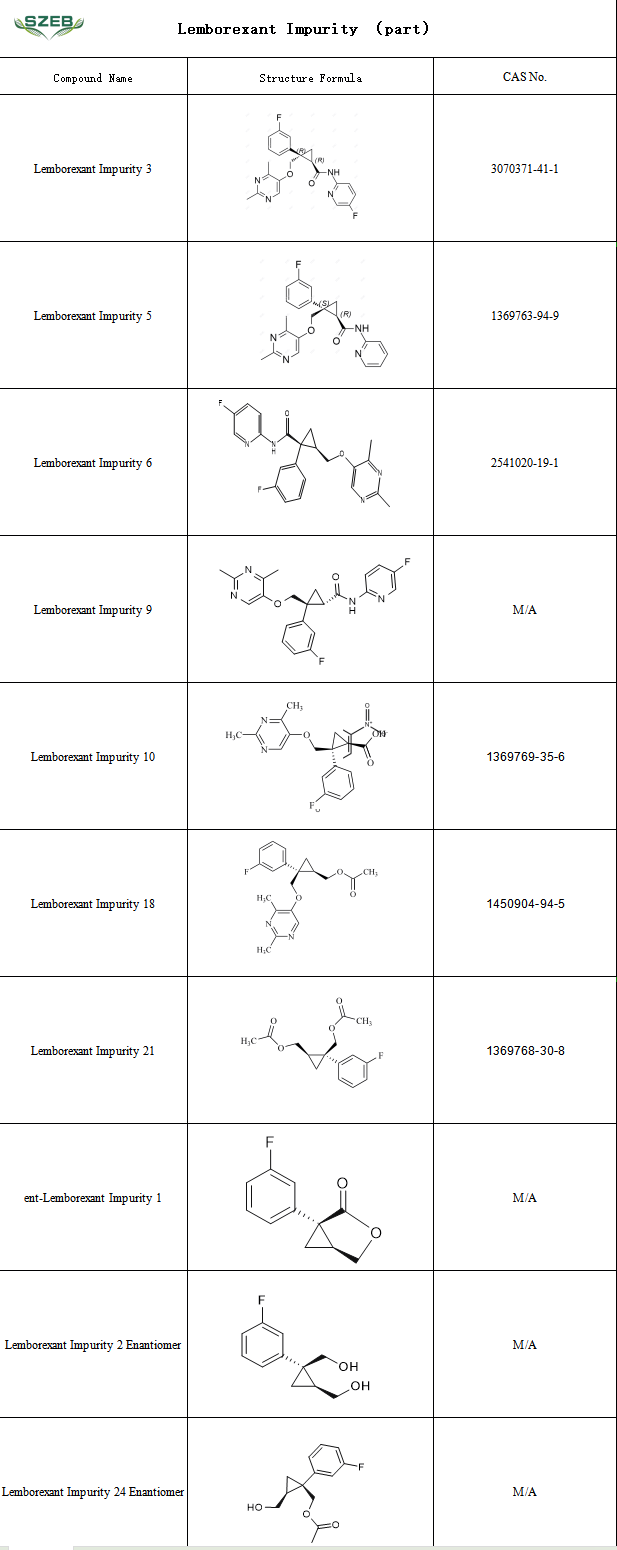
With the innovation in insomnia treatment brought by Lemborexant, the quality control of the drug becomes increasingly important, and impurity monitoring requirements will be more stringent.
SZEB advantage comprehensively supplies a full set of Lemborexant impurity reference standards in stock, providing professional support for the research, production, and quality control of pharmaceutical enterprises. Below is a partial list:
For more details on impurities, please visit our official website: www.ex-biotech.com . Welcome to contact us via email at sales@ex-biotech.com






.jpg) Wechat
Wechat