Avibactam impurity
Recently, the novel antibacterial agent Emblaveo® (Aztreonam and Avibactam Sodium for Injection), developed by Pfizer Inc., has officially received market approval from the China National Medical Products Administration (NMPA)
This is the first β-lactam antibiotic/β-lactamase inhibitor combination capable of covering all enzyme types of CRE (Carbapenem-Resistant Enterobacteriaceae). It is indicated for the treatment of adult patients with complicated intra-abdominal infections (cIAI) and hospital-acquired pneumonia (HAP), including ventilator-associated pneumonia (VAP), caused by Gram-negative bacteria
Avibactam is a novel non-β-lactam β-lactamase inhibitor. It is typically combined with specific antibiotics (e.g., ceftazidime or aztreonam) to form fixed-dose combination products. Its mechanism of action involves protecting the co-administered antibiotic from hydrolysis and destruction by β-lactamases produced by resistant bacteria, thereby restoring and enhancing the antibacterial activity of these antibiotics against resistant strains. This makes its combination products (e.g., ceftazidime/avibactam) a crucial option for treating infections caused by multidrug-resistant bacteria, such as carbapenemase-producing Enterobacteriaceae (CRE)
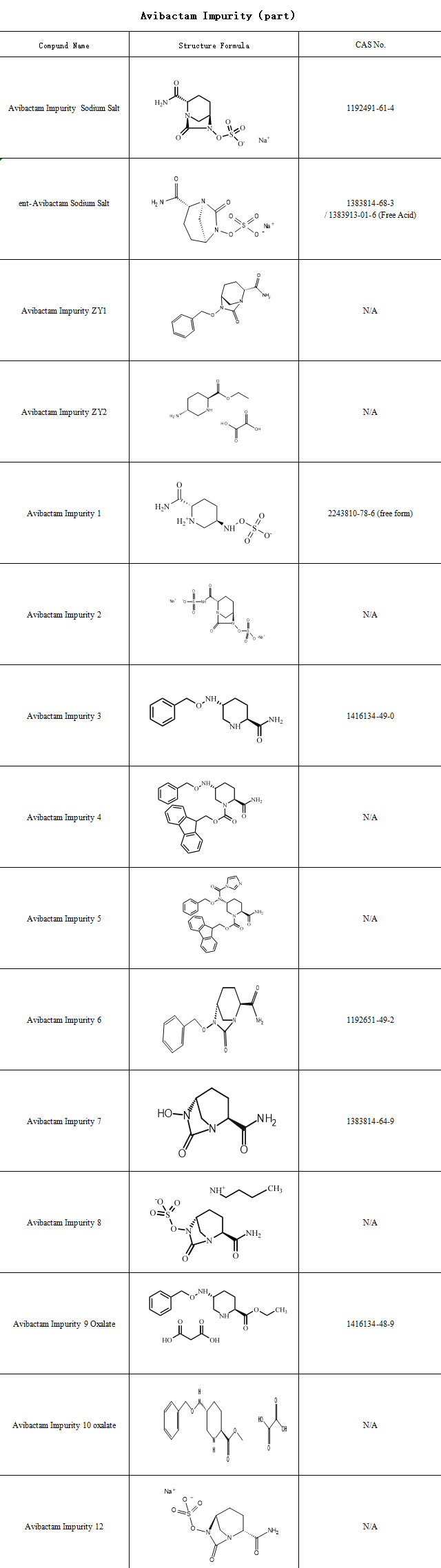
With the widespread application and in-depth research of avibactam, the analysis and control of its impurities and content have become increasingly important. During the synthesis and production of avibactam, the generation of various impurities and degradation products is inevitable. Some of these impurities may possess potential toxicity or side effects, impacting the drug‘s efficacy and safety
SZEB specializes in providing impurity reference standards for pharmaceuticals, supplying avibactam impurity reference standards to support pharmaceutical companies in their drug development and production efforts. Below is a partial list of avibactam impurities:
For more details on impurities, please visit our official website: www.ex-biotech.com
Hotline: +86 755 2305 1186
Email: sales@ex-biotech.com






.jpg) Wechat
Wechat