Loxoprofen Impurity
Time:2025-07-04
Views:81
Loxoprofen is a nonsteroidal anti-inflammatory drug (NSAID) with antipyretic, analgesic, and anti-inflammatory properties. It exerts its effects by inhibiting cyclooxygenase (COX) enzymes, thereby reducing prostaglandin synthesis to alleviate pain and inflammation while lowering fever. Loxoprofen is commonly prescribed for rheumatic diseases, arthritis, acute pain (post-surgical, post-dental extraction, headache, dysmenorrhea), and upper respiratory tract infections. It demonstrates strong efficacy, particularly in pain management.
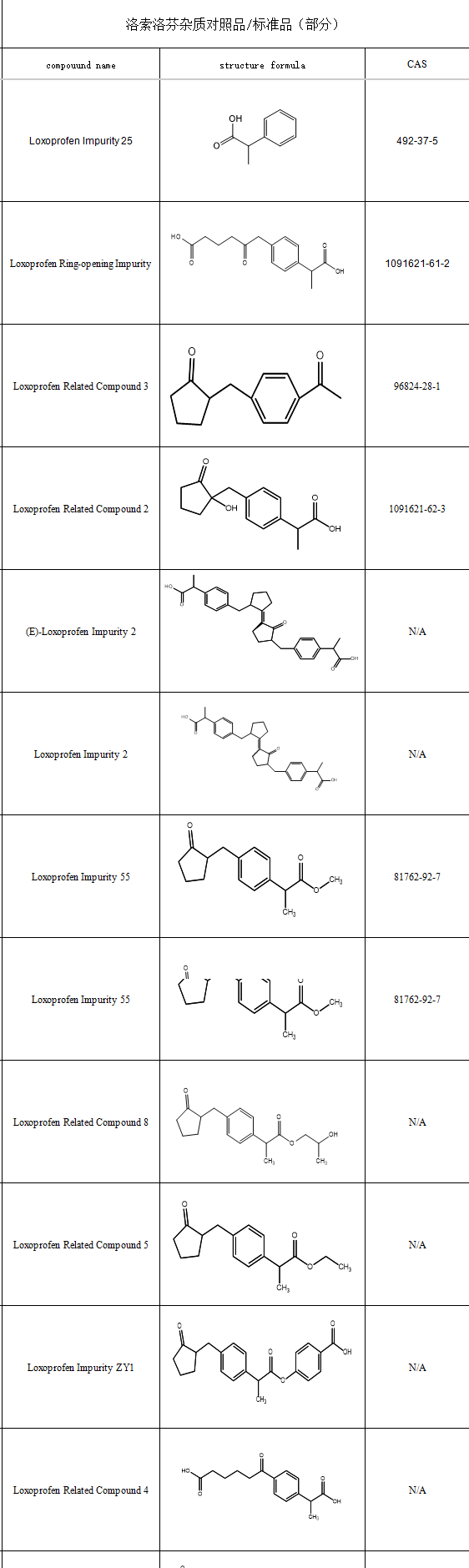
To ensure the therapeutic potency of loxoprofen, impurity control is critical for the quality of related formulations. Given its diverse synthetic pathways, loxoprofen carries multiple process-related impurities. Common impurities in loxoprofen sodium include:
o-Chlorobenzoic acid
4-Chloroaniline
4-Chlorophenylacetic acid
Loxoprofen methyl ester
Loxoprofen ethanolamine salt
Notably, brominated intermediates from synthesis may carry potential toxicity, while impurities such as ring-opened degradants and glycerol-esterified byproducts can compromise drug efficacy or even induce adverse effects. These may arise during both active pharmaceutical ingredient (API) synthesis and formulation processes.
SZEB supplies a comprehensive range of loxoprofen impurity reference standards* to support end-to-end quality control from R&D to manufacturing. Our portfolio spans residual synthetic intermediates to degradation products(Each product includes full COA & analytical data: H-NMR, MS, HPLC).Partial Catalog Highlights:
Explore our impurity library: http://www.ex-biotech.com
Contact: sales@ex-biotech.com






.jpg) Wechat
Wechat