Vitamin K impurity
Time:2025-06-20
Views:92
Vitamin K, a fat-soluble vitamin clinically known as the "Coagulation Vitamin", is primarily used to treat coagulation disorders (e.g., hemorrhagic diseases) and osteoporosis caused by vitamin K deficiency.
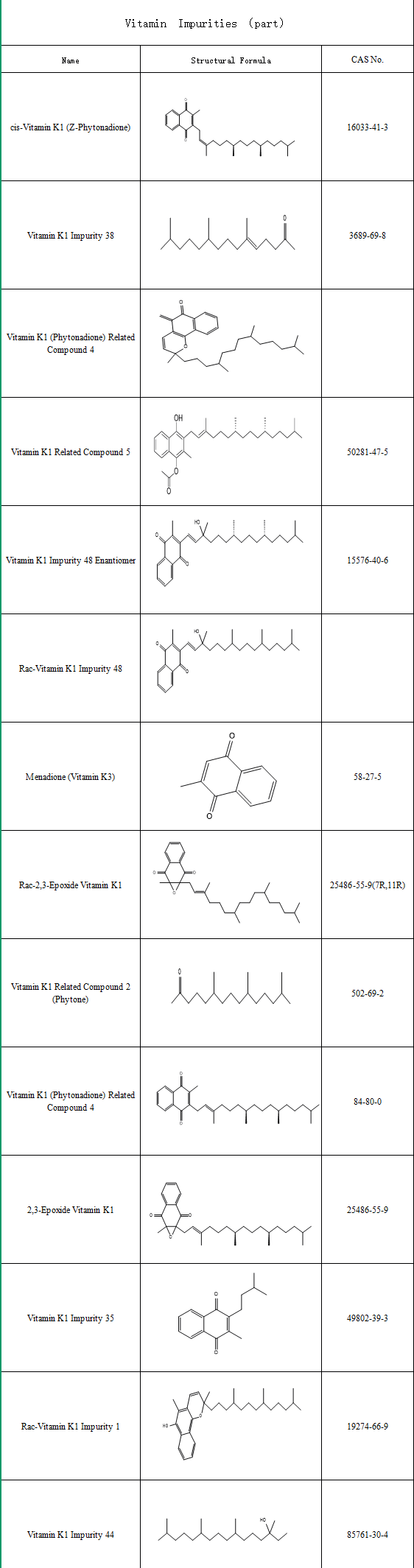
With advancing research, vitamin K—particularly the K2 subtype—has revealed multidimensional physiological functions beyond coagulation, driving increased demand for related pharmaceuticals. Consequently, stringent quality control is critical. During synthetic production, common process-related impurities include:
Stereoisomers: e.g., Vitamin K1 Impurity 8 (CAS: 16033-41-3)
Intermediate residues
Degradation impurities from light/heat sensitivity (e.g., 2,3-Epoxy Vitamin K1, CAS: 25486-55-9)
To address pain points in procurement, SZEB offer a full life-cycle management program:
We supplies a complete range of Vitamin K impurities, including:Vitamin K1 Impurity Series & Vitamin K2 Impurity Series
Traceability Documentation Provided:
COA (HPLC/LC-MS purity, water content, residual solvents)
Structural Confirmation: 1H/13C NMR, HRMS, IR, XRD
We also have flexible packaging including R&D-scale: 10mg/25mg etc,.to meet different experimental needs.
The following is a partial catalog of Vitamin K impurities, for more details of impurities, please visit the official website: www.ex-biotech.com , or you can contact us by e-mail:sales@ex-biotech.com , we are happy to serve you.






.jpg) Wechat
Wechat