special recommened——Crisaborole Impurity
Time:2024-11-08
Views:161
Crisaborole is a nonsteroidal phosphodiesterase 4 (PDE-4) inhibitor used primarily for the treatment of mild to moderate atopic dermatitis. Its formulation, Crisaborole ointment (trade name Eucrisa), is the first prescription drug of a new molecular entity approved by the U.S. FDA in the past 15 years for the treatment of atopic dermatitis (AD) and the first non-steroidal topical drug to inhibit PDE4 in the skin.Eucrisa‘s indications are being further expanded from the treatment of mild-to-moderate atopic dermatitis in paediatric patients with a minimum age limit of 2 years to 3 months of age, and is the only steroid-free topical prescription drug approved by the US FDA for paediatric patients with mild to moderate AD as young as 3 months of age.
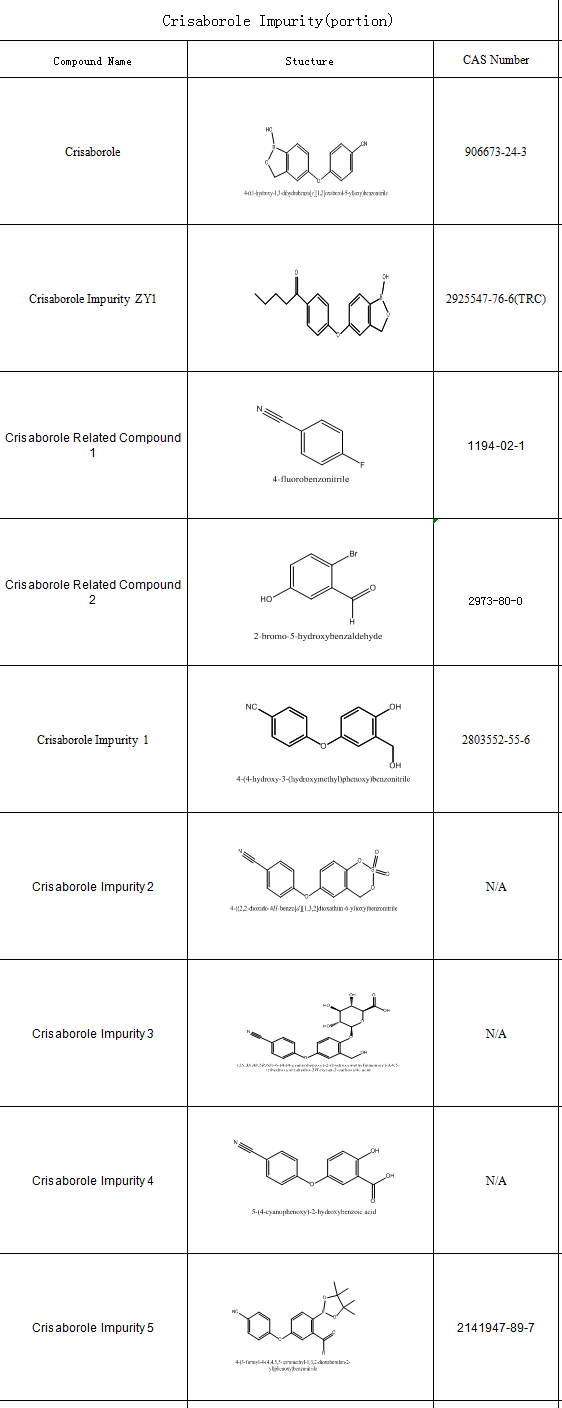
The control and detection of impurities is a key part of the drug development and manufacturing process, which helps to improve drug efficacy and reduce adverse reactions. SZEB supplies a full set of Crisaborolel impurity ,each products with COA and corresponding test certificates. Below is the catalogue of Crisaborole impurity (part), more impurity information please go to the official website: https://www.ex-biotech.com/ to view.






.jpg) Wechat
Wechat