-
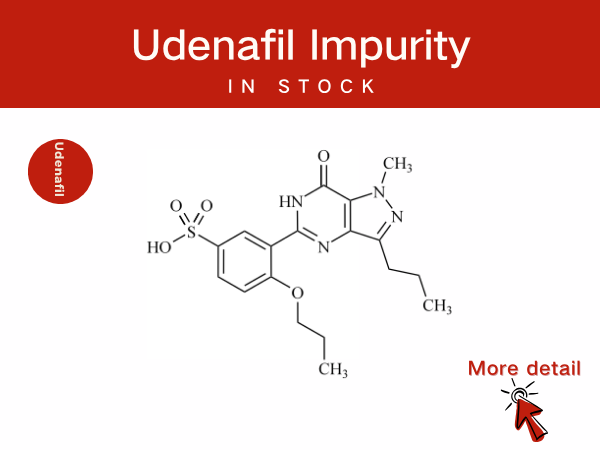
Udenafil Impurity Control and Scientific Innovation: Professional Pharmac...
Udenafil is a selective phosphodiesterase type 5 (PDE5) inhibitor. It is primarily used for the treatment of male erectile dysfunction (ED) and is also applied in the management of premature ej...
See More
-
China’s First Domestically Developed Dulaglutide Injection Approved for ...
On August 8, Boan Biotechnology announced that its independently developed Boyouping® (Dulaglutide Injection) has received market approval from the National Medical Products Administration...
See More
-
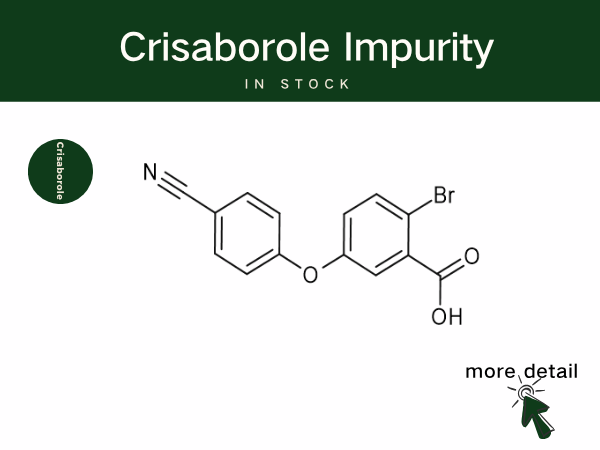
Crisaborole is Reshaping Atopic Dermatitis Treatment, Backed by SZEB Q...
Crisaborole is a boron-containing small-molecule anti-inflammatory drug, classified as a non-steroidal topical phosphodiesterase-4 (PDE-4) inhibitor. By inhibiting PDE-4, the drug increases th...
See More
-
Qilu Pharmaceutical Co.,Ltd.’s Carfilzomib for Injection Approved for M...
Qilu Pharmaceutical Co.,Ltd’s Carfilzomib for Injection has received market approval from the National Medical Products Administration (NMPA) of China for the treatment of adult patie...
See More
-
First-in-Class: FDA OKs Triple Therapy to Treat Hypertension...
Recently, George Medicines announced that its new drug Widaplik® (containing telmisartan, amlodipine, and indapamide, previously known as GMRx2) has been approved by the U.S. Food and Drug...
See More
-
LEQEMBI IQLIK™, the first-ever at-home subcutaneous injection for A...
The U.S. FDA has approved the lecanemab-irmb subcutaneous injection (U.S. brand name: LEQEMBI IQLIK™), jointly developed by Biogen and Eisai, for maintenance therapy in early A...
See More
-
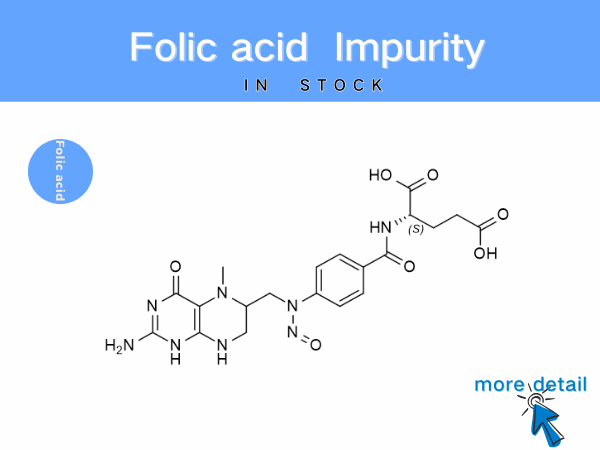
Folic Acid Impurities: Reference Standards for Pharmaceutical Quality Co...
Beyond process-related impurities, factors such as light exposure, high temperatures, or humid conditions can cause the oxidation or degradation of folic acid, leading to the formation of new impurit...
See More
-
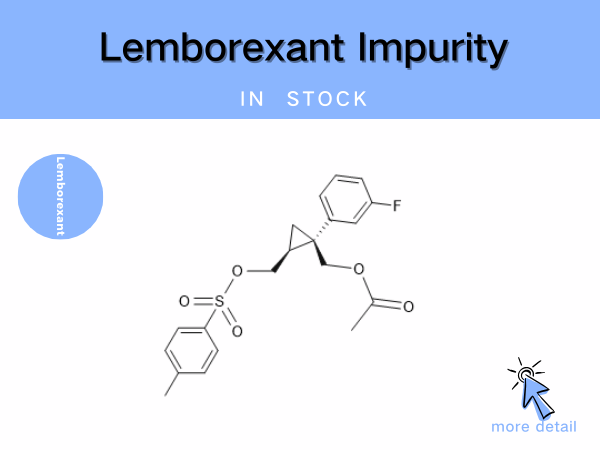
Lemborexant Impurity
Lemborexant is the first dual orexin receptor antagonist (DORA) approved for marketing in China. Developed by Eisai, it was first launched in the United States in December 2019 and received...
See More
-
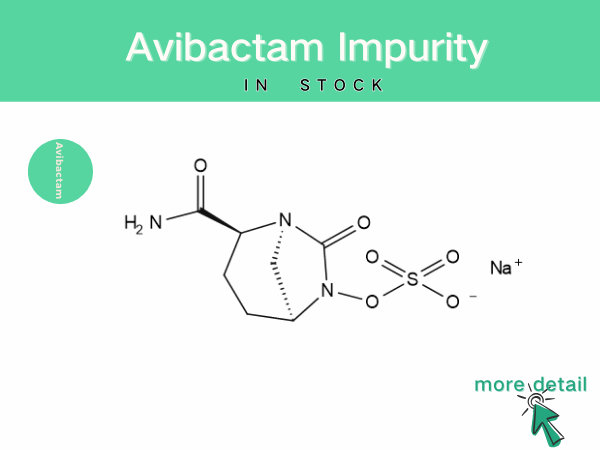
Avibactam impurity
With the widespread application and in-depth research of avibactam, the analysis and control of its impurities and content have become increasingly important. During the synthesis and production of ...
See More
-
SZEB | Continuous Updates on Nitrosamine Impurities...
Facing dynamic regulatory updates and the structural diversity of NDSRIs, Shenzhen Superior Excellence Biotechnology (SZEB) responds rapidly by supplying relevant drug impurity refer...
See More
-
Breaking Dosage Form Barriers: Escitalopram Oxalate Drops Approval ...
the Escitalopram Oxalate Drops of Guangzhou Yipinhong Pharmaceutical Co., Ltd successfully obtained the drug registration certificate issued by the National Medical Products Administration...
See More
-
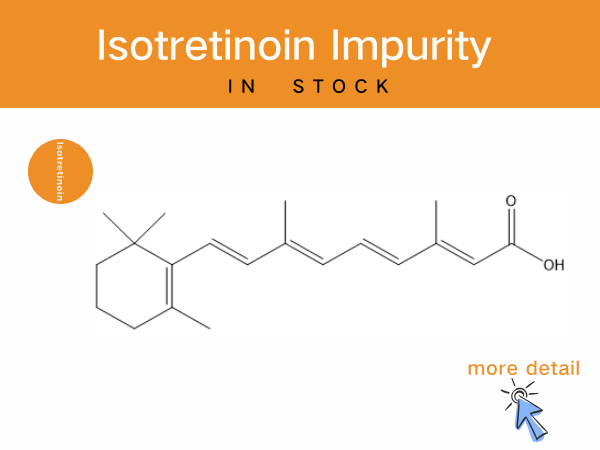
Isotretinoin Impurities: Professional Solutions of Reference Standards for...
As a critical drug for moderate-to-severe acne, Isotretinoin’s production and quality control are subject to rigorous regulatory standards. The Chinese Pharmacopoeia (2025 Edition)mandates that...
See More
-
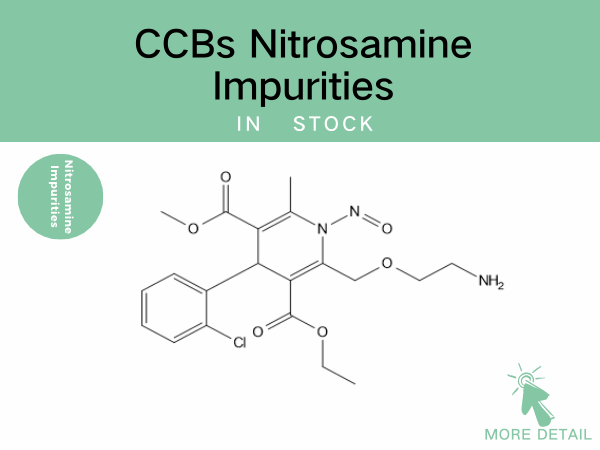
CCBs Nitrosamine Impurities——Precise Drug References Empowering Drug...
Nitrosamine impurity control remains a global regulatory priority. For CCBs—the most widely prescribed antihypertensive class—robust identification and quantification of these impurities are cr...
See More
-
the first approval of an oro-dispersible film dosage form in China...
Qilu Pharmaceutical’s risperidone oro-dispersible film has been formally approved for market launch by the National Medical Products Administration (NMPA), marking the first approval of an ...
See More
-
How Does Olopatadine Safeguard Allergy Patients?...
Impurity control is critical for drug efficacy and safety. Olopatadine may generate impurities during synthesis, storage, or metabolism. The Chinese Pharmacopoeia (2025 Edition) mandates limits ...
See More
-
J&J Submits Oral IL-23 Peptide Drug for FDA Approval: Sustained...
Global pharmaceutical giant Johnson & Johnson (J&J) and Protagonist Therapeutics recently announced the formal submission of a New Drug Application (NDA) to the U.S. Food and Drug Ad...
See More
-
Seloxavir Marboxil Tablets Approved: The Quality Control System Behind ...
On July 18, the domestic self-developed anti-influenza oral drug Seloxavir Marboxil Tablets has been officially approved by China‘s National Medical Products Administration (NMPA) for ...
See More
-
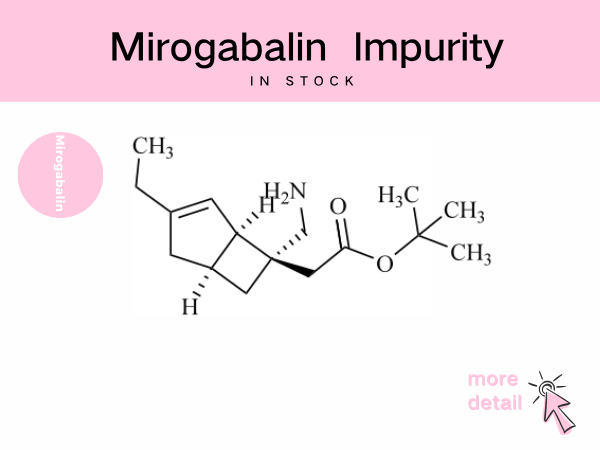
Mirogabalin Impurity ——in stock
The mirogabalin molecule contains multiple chiral centers and unsaturated bonds, rendering it susceptible to generating stereoisomers or positional isomer impurities during synthesis and storage, as ...
See More
-
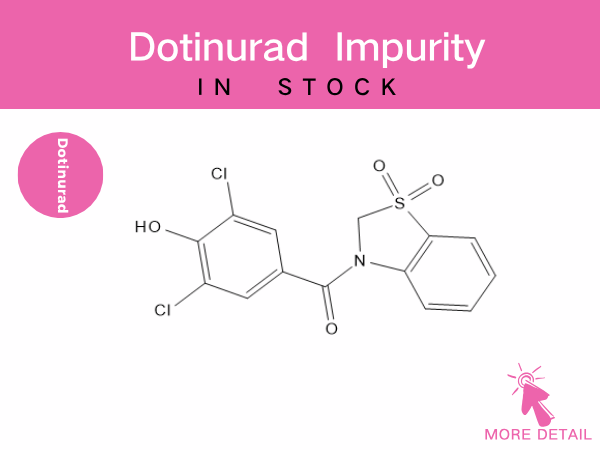
Dotinurad Related Impurities
As a novel urate-lowering drug, impurity control is a critical step in ensuring its pharmaceutical safety. Dotinurad-related impurities primarily stem from synthesis process byproducts and d...
See More
-
Mazdutide — World‘s First GCG/GLP-1 Dual-Receptor Agonist for ...
On June 27, Innovent Biologics’ Mazdutide Injection received approval from China‘s National Medical Products Administration (NMPA) for long-term weight control in adults with obesity ...
See More















.jpg) Wechat
Wechat